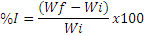INTRODUCTION
Myrciaria dubia (Kunth) Mc Vaugh, known as camu-camu, araçá-dágua, azedinho, camo camo and caçari (Smiderle and Sousa, 2008), is a fruiting plant of the family Myrtaceae, distributed throughout almost the whole of the Amazon, and commonly found in its natural state on the shores of rivers and lakes, in floodplains and in areas of flooded forest. In the state of Roraima, Brazil, it is widely distributed along the banks of rivers in the municipalities of Amajari, Boa Vista, Bonfim, Cantá, Caracaraí, Caroebe, Normandia and Rorainópolis (Chagas et al., 2015; Nascimento et al., 2019). Its fruit is frequently exploited by the local population, but not on a commercial scale (Souza et al., 2017).
The main property of the fruit of the camu-camu is its high vitamin C content, around 6112 ± 137.5 mg of ascorbic acid 100-1 g of pulp, superior to most cultivated plants (Yuyama et al., 2002a). The bark of the camu-camu can also be used, due to its important potential as a source of dietary fibre, and is one option in the diet of Amazonian peoples (Yuyama et al., 2002b).
Despite the importance of the species, there is few research on seeds of native fruit species in the Amazon, especially regarding storage and maintaining viability over long periods. However, Yuyama et al. (2011), evaluating the effect of different storage environment and forms of conservation for maintaining the viability of camu-camu seeds, concluded that the storage in water and in natural condition did not affect the germination, being able to be storage in a period of six and four months respectively.
Grigio et al. (2015), evaluating the type of packaging and the storage temperature that best preserve the quality attributes of the camu-camu found that the quality and ascorbic acid content attributes were preserved for longer in fruits stored in expanded polystyrene trays covered with PVC film at 15 °C.
Previous search of to maintain their physiological quality and vigour during storage, the seeds must be stored safely and correctly (Smiderle et al., 2018; Souza et al., 2019). Due to the difficulty of germinating seeds of species native to the north of Brazil, alternative tests such as the imbibition curve and electrical conductivity test are being applied in seed laboratories under controlled conditions in order to define the vigour and viability of the seeds (Oliveira et al., 2016).
Speed in obtaining reliable results is one of the main aspects to be considered when evaluating seed quality, as it allows to make decisions promptly and on a larger scale, reducing the risks and costs of operations such as harvesting, processing, storage and marketing (Menegatti et al., 2017; Dias et al., 2019).
In the test of electrical conductivity, the values obtained are due to disorganisation of the cell membrane and a reduction in respiratory and biosynthetic capacity (Castilho et al., 2019). Given these two processes, electrolytes are released into the immersion water, and the intensity of their electric current measured, both by weight and individually (Silva et al., 2019).
However, considering that seedling vigour is one of the main economic components of a production system of fruit seedlings, an analyse of characteristics that may indicate the physiological quality and vigour of seeds of Myrciaria dubia is of paramount importance, as these can serve as indicators for obtaining early better-quality seedlings, as well as aiding in suggesting and selecting superior new genotypes.
Given the above, the study aimed to investigate the physiological quality of Myrciaria dubia (Kunth) McVaugh seeds, stored for 300 days in different conditions and packaging, using tests of electrical conductivity and seedling vigour.
MATERIAL AND METHODS
Plant material
The present work was carried out at the Seed Analysis Laboratory and seedling nursery of the forestry sector of Embrapa Roraima, Brazil. Ripe fruits of Myrciaria dubia were collected from the banks of the River Jatapú (0º41'072” N and 59º18'046” W, at an altitude of 144 m), in Caroebe, in the state of Roraima, and taken to the seed laboratory of Embrapa Roraima for the experiment.
Seeds preparation
First, the fruits were pulped to remove the seeds. The seeds were then washed in running water until the residue was eliminated. The seeds were then disinfected by washing in running water, and treated for five minutes with 0.25% sodium hypochlorite solution diluted in 100 mL of water. Intact seeds of Myrciaria dubia were separated into three weight classes according to Nascimento et al. (2019). Small seeds were considered those with a weight of less than 0.84 g, medium seeds with a weight between 0.86 g and 1.20 g, and large seeds, a weight of greater than 1.22 g. Measurements were taken individually on a precision scale (0.001 g) in four replications, and 50 seeds per replication as samples (200 seeds/ weight class).
Imbibition by seeds of Myrciaria dubia
The water content of the seeds was determined in a greenhouse (105±3ºC) for 24 hours for each weight class, as per the procedure described by Brasil (2009), in five replications of 10 seeds.
Seed imbibition was carried out in four replications of 50 seeds for each class. The imbibition process was monitored by periodic weighing after 0, 6, 12, 24, 48, 72, 96, 120, 144, 168, 192 and 216 hours. After the final weighing, the seed moisture content was determined (Brasil, 2009). For each weighing, the percentage of seed imbibition (%I) was determined using the formula:
The values obtained for imbibition were submitted to regression analysis at 5% probability in a completely randomised design and a 3 x 12 factorial experiment (three seed classes and 12 weighing periods).
Evaluation of seeds quality
For the comparative evaluations of seed physiological quality during storage, a mean seed-weight class was established. For each storage period (0, 30, 60, 120, 180, 240 and 300 days) a sample of 110 seeds was taken from each container and submitted to the following evaluations: imbibition curve, seed dry weight, electrical conductivity, seedling emergence (%SE) and speed of emergence index (SEI).
In addition, the electrical conductivity (EC) test was carried out in a completely randomised design as factorial experiment of 2 environments x 2 types of packaging x 7 time (EC), in five replications of 10 seeds each. For this purpose, the seeds were placed in plastic pots of 200 mL capacity containing 75 mL of distilled water according to Oliveira et al. (2016). The material was then placed in a Biochemical Oxygen Demand incubator (BOD) set at 25°C.
EC readings were taken for each storage period (0, 30, 60, 120, 180, 240 and 300 days). The seeds were placed in individual packaging to be stored for up to 300 days in the laboratory at 23-25°C and an RH of 60-70%, and in a cold chamber at 14-16°C and an RH of 50-60%. The packaging consisted of glass containers and plastic pots topped up with deionized water, with the water exchanged at regular intervals of seven days.
After each storage period, the electrical conductivity of the imbibition solution was determined using a conductivity meter (model MCA 150-country China), with the results expressed as μS cm-1 g-1 seed. The seedling emergence test was carried out in order to complement and clarify the results obtained with the electrical conductivity test.
Evaluation of seedling emergence
The tests of seedling emergence were carried out in a greenhouse in a seedbed containing 50% sand + 50% sawdust (v:v; 1:1) as substrate, with the seeds sown at a depth of 2.0 cm. Substrate moisture was maintained by manual irrigation four times daily. The experimental design was completely randomised with subdivided plots, where the plots comprised a 2 x 2 factorial scheme (two types of packaging x two environments) and the subplots comprised the seven periods of evaluation. The germination tests were evaluated daily until the count stabilised (Laboriau, 1983). From the data obtained in the test of emergence, the speed of emergence index (SEI) was calculated as per Maguire (1962).
Statistical analysis
All the data were tested for homogeneity of variance (Bartlett) and normality (Shapiro-Wilk). The mean values of the variables under evaluation were submitted to analysis of variance and, when significant, to the mean-value comparison test (Tukey) at 5% probability. Regression analysis was carried out for the factor time (months). The statistical analysis was performed with the aid of the Sisvar software -version 5.4 (Ferreira, 2011).
RESULTS AND DISCUSSION
The results of the study for the seed classes of Myrciaria dubia showed a increase in the initial moist weight up to 72 hours, which varied according to each seed class. Small seeds had an increment of 8.5%, followed by large seeds with an increment of 7.9%, when compared to the beginning of the imbibition process (Figure 1). This configuration is because the seeds before imbibition have a negative water potential: when in contact with water, the first phase is of rapid absorption due to the difference in potential between the seed and the medium.
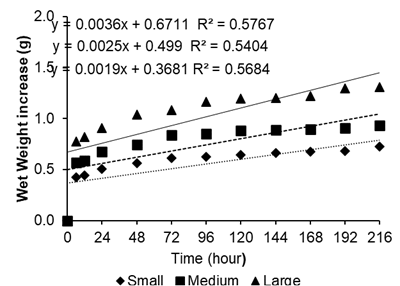
Figure 1 Increase in seed moist wet weight (g) by the three seed classes of Myrciaria dubia (Kunth) McVaugh over 216 hours.
According to Oliveira et al. (2016), the imbibition process is initially characterised by rapid water absorption and an accelerated increase in the water potential of the embryo, followed by a marked reduction in the rate of water imbibition. This was found in the present study.
After 72 hours of immersion in water, imbibition stabilised up to 216 hours for seeds of the different weight classes of Myrciaria dubia, suggesting the existence of a survival strategy for the camu-camu that makes the coat less water-permeable, given that the fruit of Myrciaria dubia ripens during the period of river flooding, and seed dispersal is endozoochoric, mainly by fish, such as the tambaqui (Colossoma macropomum), and by the current of the watercourses (Yuyama and Siqueira, 1999; Yuyama, 2011).
After the period of increasing moist weight of up to 216 hours, the second stage of the experiment began for a comparative evaluation throughout the storage period of the physiological quality of the seeds classified as medium. The results of the variance analysis and the mean values of the characteristics evaluated during the physiological quality of the seeds and the test of seedling emergence are shown in Table 1. Estimates of the experimental coefficients of variation ranged from 2.20% for electrical conductivity (EC) and up to 13.02% for the speed of emergence index (SEI).
Table 1 Variance analyses of the water imbibition curve (IC), electrical conductivity (EC), seedling emergence (SE) and speed of emergence index (SEI) in seeds of Myrciaria dubia (Kunth) McVaugh stored in different packaging and conditions
| SV | DF | MS | |||
| IC | CE | SE | SEI | ||
| Repl | 3 | 0.191675 | 0.002918 | 37.202381 | 0.008218 |
| Conditions | 1 | 3.281151 | 0.208294** | 228.571429 | 0.020901 |
| Imbib | 1 | 1.397322 | 0.090858** | 0.892857 | 0.009844 |
| Condit*Imbib | 1 | 2.103772 | 0.95472** | 3.571429 | 0.007072 |
| Error 1 | 9 | 1.130518 | 0.007014 | 90.972222 | 0.008525 |
| Period | 6 | 159.101** | 15.290379** | 321.354167** | 1.512160** |
| Period*Condit | 6 | 3.605703** | 0.028031** | 43.675595 | 0.006988 |
| Period*Imbib | 6 | 0.778824 | 0.039091** | 138.913690** | 0.011477* |
| Period*Condit*Imbib | 6 | 0.792208 | 0.025664** | 81.175595 | 0.004551 |
| error 2 | 72 | 0.912546 | 0.003674 | 38.640873 | 0.005197 |
| Mean | 43.47 | 1.03 | 90.62 | 0.71 | |
| CV 1 (%) | 2.45 | 8.17 | 10.52 | 13.02 | |
| CV 2 (%) | 2.2 | 5.91 | 6.86 | 10.16 | |
**,*- Significantt by F-test at 1% and 5% probability, respectively.
The results for the imbibition curve revealed significant differences for storage period and a significant interaction between the storage period and the environment. Figure 2A shows a strong relationship, greater than R2 = 0.88**, as a function of the environment and storage period. In the study under analysis, the storage conditions presented oscillations during each period, throughout the 300 days.
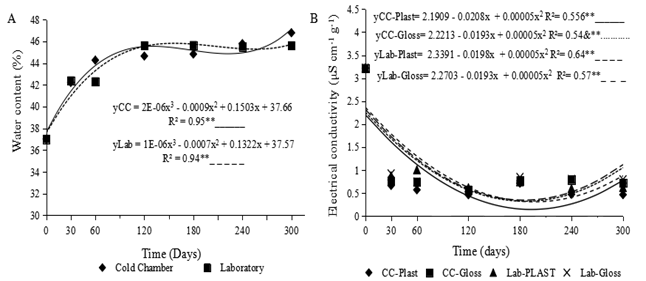
Figure 2 Imbibition curves (A) and electrical conductivity (B) in seeds of Myrciaria dubia (Kunth) McVaugh stored in a cold chamber (CC) or laboratory (LAB) conditions (A and B), in Plastic (Plast) or Glass (Glass) packaging (B). ** - Significant effect by F-test at a level of 1% probability.
The seeds stored in the cold chamber showed a tendency to adapt to the three-phase absorption pattern proposed by Bewley and Black (1994), as there was a rapid increase in water content up to 60 days of storage. The beginning of phase II, characterised by a stationary period of absorption, continued for up to 240 days, when an increase in the water content was again seen, indicating the beginning of phase III, however without root emission. The seed water content in the laboratory was found to increase up to 120 days of storage, remaining constant until the end of the experiment at 300 days.
It was found that the greatest weight loss in the camu-camu seeds occurred up to 60 days of storage (Figure 3); whereas throughout the storage period (300 days), the overall loss in seed weight was 16.8% (Figure 3).
The test for electrical conductivity in seeds of Myrciaria dubia shown in Figure 2B, indicates a significant effect (P<0.01) for the package x conditions x storage period (Table 1).
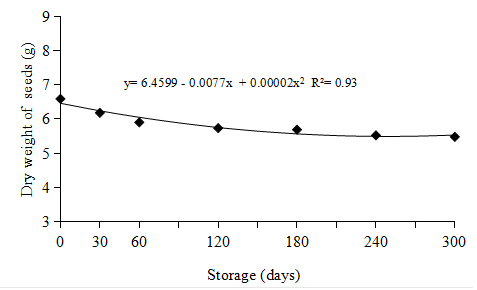
Figure 3 Dry weights of seeds (ten seeds) over the 300 days of storage in of Myrciaria dubia (Kunth) McVaugh.
A breakdown of the interaction between packaging and environment for electrical conductivity within each storage period showed a quadratic polynomial fit (Figure 2B). The highest mean value for electrical conductivity was found during period zero, i.e. during the initial evaluation (Figure 2B) demonstrated that the values for electrical conductivity in the different environments and packaging during the storage periods showed a reduction, with the lowest value being seen for the plastic pots stored in the cold chamber, of 0.028 μS cm-1 g-1 at 216 days of storage. For the plastic packaging stored under laboratory conditions, the EC value was 0.379 μS cm-1 g-1at 196 days, while for the glass containers under laboratory conditions this value was 0.408 μS cm-1 g-1 and in the cold chamber, 0.359 μS cm-1 g-1.
An inverse relationship was found when correlating the imbibition curves in seeds of Myrciaria dubia (Figure 2A) with electrical conductivity (Figure 2B) as the water content increased the conductivity decreased. This can be explained by reorganisation of the cellular content and the smaller release of electrolytes, as shown in Table 2 during imbibition, the membrane system of the seed reorganises and reacquires control of permeability. Superiority in the seed imbibition curve and electrical conductivity was seen at 240 days. This is explained by the beginning of absorption phase III, which causes the integument to rupture, with a greater release of solutes into the solution. Table 2 shows the values of electrical conductivity in seeds of Myrciaria dubia stored for 300 days in plastic and glass containers in a cold chamber and laboratory environment.
Table 2 Mean values for electrical conductivity (μS cm-1 g-1) in seeds of Myrciaria dubia (Kunth) McVaugh stored for 300 days in a cold chamber (CC) or laboratory (LAB) conditions in packaging of plastic pots and glass containers
| Storage | Packaging | |||||||
| Time (Days) | Conditions | Plastic | Glass | |||||
| 0 | CC | 3.217 | a | A | 3.217 | a | A | |
| Lab | 3.217 | a | A | 3.217 | a | A | ||
| 30 | CC | 0.665 | a | A | 0.757 | a | B | |
| Lab | 0.875 | b | A | 0.922 | b | A | ||
| 60 | CC | 0.570 | a | A | 0.740 | a | B | |
| Lab | 1.007 | b | B | 0.740 | a | A | ||
| 120 | CC | 0.462 | a | A | 0.545 | a | B | |
| Lab | 0.615 | b | A | 0.572 | a | A | ||
| 180 | CC | 0.715 | a | A | 0.772 | a | A | |
| Lab | 0.745 | a | A | 0.845 | a | B | ||
| 240 | CC | 0.472 | a | A | 0.790 | a | B | |
| Lab | 0.600 | b | A | 0.802 | a | B | ||
| 300 | CC | 0.365 | a | A | 0.395 | a | A | |
| Lab | 0.420 | a | A | 0.420 | a | A | ||
Mean values followed by different lowercase letters in a column and uppercase letters on a line, differ by Tukey’s test at 5% probability level. CC = cold chamber and LAB = laboratory.
At 30, 60, 120 and 240 days of storage, there were significant differences in electrical conductivity between the laboratory and cold-chamber conditions for the plastic packaging (Table 2), while there were no significant differences between the periods of 60, 120 or 240 days of storage for the glass containers. However, it was found that the storage periods of 60 and 240 days for both conditions and both types of packaging showed significant differences in electrical conductivity. When comparing the packaging at 60 days, seeds stored in glass and kept in the laboratory showed lower conductivity than the seeds in plastic pots. For the remaining periods, the seeds stored in plastic pots showed lower conductivity than those in the glass containers.
The results for conductivity were low, probably due to the intrinsic characteristics of the species, which shows high cellular organisation, or due to the high water content of the seeds. Certainly, the combination of temperature and high relative humidity provided more favourable conditions, which minimised the speed of seed deterioration. According to Souza et al. (2019), the aim of storage is to keep the seeds in cryptobiosis, i.e. in a state of embryo arrest through a reduction in active metabolism.
The result obtained for different storage periods and packaging demonstrates, with the test for seedling emergence, a slightly decreasing linear trend (Figure 4A) over each period (days). Up to 120 days of storage, the seeds in the glass packaging presented a higher percentage emergence than those in the plastic containers. From 240 days onwards, the seeds stored in plastic containers showed a higher percentage of emergence than those in the glass packaging (Figure 4A). However, the value for percentage of emergence until the end of the experiment kept the value above the minimum of 85% established for the market (Brasil, 2009).
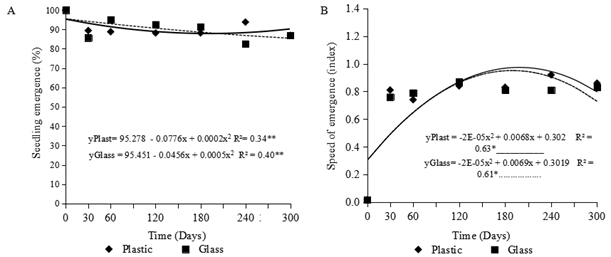
Figure 4 (A) Seedling emergence (%) and (B) speed of emergence (SEI, index) in seeds of Myrciaria dubia (Kunth) McVaugh stored for 300 days in glass containers and plastic pots.
The SEI showed a quadratic trend for both the plastic and glass containers (Figure 4B), with the greatest indices seen at 170 days for both types of packaging. However, the plastic packaging gave a higher emergence index throughout almost the entire experiment, with the speed of emergence index being higher in the glass packaging after approximately 220 days of storage.
Throughout the storage period, the water content of the seeds ranged from 37 to 47%, which is lower than that reported by Yuyama et al. (2011), however, there was no effect on the percentage of emergence of the camu-camu seedlings.
The values for seedling emergence were similar to those reported by Ferreira and Gentil (2003), when evaluating the storage of camu-camu seeds at different levels of humidity. However, when the temperature was verified, only the seeds stored at 10°C at 43% humidity continued viable for up to 280 days, with 81% germination (Nascimento et al., 2011).
Yuyama et al. (2011) reported over 90% germination in seeds with no pulp, and seeds washed/treated with hypochlorite and stored in water for up to six months, or in a natural environment for up to four months.
It is therefore important to store the seeds of Myrciaria dubia in plastic or glass containers, since they are of low cost, and storage can be carried out manually, offering the producer greater assurance regarding the physiological quality of the acquired plant material used in producing seedlings of Myrciaria dubia. Given that seed quality plays a major role in the cultivation of Myrciaria dubia, it can be considered one of the principal bottlenecks, especially in seedling production.
CONCLUSIONS
The seeds of Myrciaria dubia show a strategy for stabilising imbibition over long periods of immersion in water.
Seeds of Myrciaria dubia stored in plastic pots topped up with water in a laboratory or cold-chamber conditions are indicated for maintaining physiological quality and generating vigorous seedlings up to 300 days.