INTRODUCTION
Brazil produces about 141 million tons of soybeans (Glycine max L.), being the world's largest producer (CONAB, 2021). This great relevance increasingly increases the demand for the use of high-quality seeds, whether in their physical, physiological, genetic, and sanitary aspects, since it is a key input for the formation of uniform and productive crops (Krzyzanowski et al., 2018).
The physiological quality of soybean seeds is maximum near physiological maturity, corresponding to the R7 stage. However, seeds at this stage have high moisture, which makes it impossible to harvest them, which should be done at the R8 stage, when 95% of the pods present a brown coloration, typical of mature pods (Fehr and Caviness, 1977). In the period between physiological maturity and harvest, the seeds are exposed to several environmental factors that contribute to the reduction of their quality, such as high temperatures (Alsajri et al., 2020), excessive rainfall (Pinheiro et al., 2021; Pinheiro et al., 2023), stink bug damage (Defensor et al., 2021), among others. In general, all these factors mentioned contribute to accelerating the deterioration process, which involves several metabolic and physiological disturbances that result in reduced germination and seed vigor (Ebone et al., 2019).
In addition to reducing the physiological quality of seeds, their permanence in the field until the ideal harvest point is reached can also contribute to the increase in the incidence of seed-associated microorganisms, reducing the sanitary quality. The presence of seed-associated fungi can be one of the main causes of the reduction of seed quality in the field (Krzyzanowski et al., 2018). In soybean crop, contamination by fungi such as Penicillium spp., Fusarium spp. (Ferreira et al., 2019); Cercospora kikuchii (Krzyzanowski et al., 2019; Brzezinski et al., 2022), Phomopsis spp. (Rodrigues et al., 2019), and others, have been consistently reported to be detrimental to quality seed production. Seed contamination by these pathogens contributes not only to accelerating deterioration in the field, with negative effects on germination and vigor, but also to the dissemination and introduction of these pathogens into disease-free areas (Krzyzanowski et al., 2018).
The tolerance of soybean seeds to deterioration in the field differs among cultivars. In this sense, studies have been conducted seeking to clarify the events related to this process in an attempt to establish criteria that can be used to select genotypes with high-quality seed characteristics. Among the methods employed for this objective, the harvest delay has been widely explored. Keeping seeds in the field after the R8 stage contributes to deterioration, reducing their physiological potential, with differentiated responses depending on the genotype (Zuffo et al., 2020). For cultivar ‘Detap-1’, a delay of more than 3 days after R8 significantly compromised seed germination and vigor (Idaryani et al., 2021). Harvest delay for five and 10 days after R8 of seeds of ‘PB-1’ cultivar provided reduced germination percentage, germination index and rate, seedling vigor, and deterioration-related biochemical changes (Weerasekara et al., 2021). Soybean seeds of different cultivars harvested at 20 and 30 days showed decreases in germination and vigor (Fialho et al., 2022).
Therefore, the association of harvest delay with physiological and sanitary characteristics of seeds of different soybean cultivars may be of interest in studies on seed deterioration in the field and aid in the selection of more resistant materials. Given the above, the work aimed to assess the physiological and sanitary quality of seeds of three soybean cultivars submitted to harvest delay in the field.
MATERIAL AND METHODS
Soybean seeds of the cultivars ‘BMX Potência’, ‘NS 5959 IPRO’ and ‘TMG 1175 RR’, of relative maturity groups 6.7, 5.9, and 7.5, respectively, were produced in the experimental area of the Agronomy Department of the Universidade Federal de Viçosa, Viçosa, Minas Gerais, Brazil. The climatic data recorded in the pre-harvest and harvest stages of the seeds are presented in Figure 1 (A and B) (Fialho et al., 2022).
After soil analysis and preparation, the planting fertilization was done according to the results of soil analysis. Before sowing, seeds were treated with Derosal Plus fungicide (carbendazim + thiram) at a dose of 200 mL 100 kg-1 of seeds. Sowing (14 seeds m-1) was conducted on November 10, 2017. The useful experimental area was 79.5 m2, with four rows 53 m long, spaced 0.5 m apart. Each plot consisted of two 15 m long central rows. Three plots were used for each cultivar, totaling 15 linear meters per cultivar within each row. The borders of the experimental field were made up of soybean plants, spaced at 0.5 m from each side of the area. The cultural treatments were performed when necessary, following the instructions for the management of soybean culture.
The harvest was performed manually in four periods: i) R8 stage (95% of the pods had the typical color of mature pods); ii) R8 + 10 days; iii) R8 + 20 days and iv) R8 + 30 days. The harvest dates for each treatment and the moisture content of the harvested seeds are presented in Table 1.
Table 1 Dates of harvests in 2018 at stages R8, R8 + 10, R8 + 20, and R8 + 30 days and seed moisture content of three soybean cultivars at each harvest
| Cultivar | R8 | Moisture (%) | R8 + 10 | Moisture (%) | R8 + 20 | Moisture (%) | R8 + 30 | Moisture(%) | ||||||||||||||
| NS 5959 | Mar/23 | 28.2 | Apr/02 | 18.3 | Apr/12 | 17.3 | Apr/22 | 17.0 | Apr/06 | 31.2 | Apr/16 | 19.0 | Apr/26 | 16.9 | ||||||||
| BMX Potência | Mar/27 | 23.9 | Apr/06 | 24.8 | Apr/16 | 18.3 | Apr/26 | 16.3 | Apr/03 | 23.2 | Apr/13 | 17.8 | Apr/23 | 16.9 | May/03 | 12.9 | Apr/20 | 14.9 | Apr/30 | 15.0 | May/10 | 14.8 |
| TMG 1175 | Apr/02 | 22.5 | Apr/12 | 20.1 | Apr/22 | 17.9 | May/02 | 12.6 | Apr/10 | 27.2 | Apr/20 | 14.7 | Apr/30 | 14.5 | May/10 | 15.0 |
Moisture content
Three replications of 25 seeds were used. The moisture content was determined by the oven method at 105 ± 3 oC for 24 h, with the results expressed in percentage (wet basis) (Brasil, 2009).
Germination
Four replications of 50 seeds were used and sown on paper towels (Germitest®) moistened with a volume of water equivalent to 2.5 times the weight of dry paper. Rolls were made and kept in a germinator at 25 °C. Assessments were performed on the eighth day after sowing and the results were expressed in percentage of normal seedlings (Brasil, 2009).
Seedling emergence
It was conducted in a greenhouse with four replications of 50 seeds for each treatment. The seeds were placed to germinate in Styrofoam trays, containing a mixture of soil and sand in the proportion 2:1 (v/v) moistened until it reached 60% of its water retention capacity. During the test, irrigations were performed whenever necessary to maintain the moisture close to field capacity. Daily counts were made until the number of emerged seedlings with cotyledons above the surface of the substrate stabilized. The results were expressed as a percentage of emerged seedlings (Krzyzanowski et al., 2020).
Seedling image analysis by Vigor-S®
Four replications of 20 seeds were used per treatment. The seeds were distributed, with hilum facing the bottom of the paper, in a line drawn on the upper third of two sheets of towel paper and covered with a third sheet. The paper was previously moistened with an amount of water equivalent to 2.5 times its dry weight. The rolls were made and placed vertically in a germinator at 25 °C for 3 days. After this period, the seedlings were transferred to a blue cardboard sheet with an area of 30 cm x 20 cm. Seedling images were acquired by scanning in a scanner (HP, Scanjet 200) fixed upside down inside an aluminum box (60 × 50 × 12 cm), set to 300 dpi resolution, and coupled to a computer. The images obtained were processed individually by the Vigor-S® program, installed in a computer, which generated the variables vigor index and uniformity index (Rodrigues et al., 2020).
Sanity
The filter paper method or "blotter test" was used, with four replications of 25 seeds on four sheets of sterilized germ paper moistened with distilled and autoclaved water in "gerbox" type boxes. The incubation was done in a B.O.D, at 25 ºC, with 12 hours of lighting with fluorescent lamps, alternated with 12 hours of darkness, for seven days. After this period, the fungi present in the seeds were identified, with the help of magnifying glass with illumination and stereoscopic microscope (Goulart, 2018). The results were expressed as percentage of infected seeds.
Experimental design and statistical analysis
The completely randomized design was used in a double factorial scheme 3 (cultivars) x 4 (harvesting periods), with four replications. The data were submitted to analysis of variance (ANOVA). The normality of the data was tested by the Shapiro-Wilk test and homoscedasticity by Bartlett's test. The means were compared by Tukey's test (p ≤ 0.05). Multivariate principal component analysis (PCA) was also performed. Software (R Core Team, 2022) was used for all statistical analyses.
RESULTS AND DISCUSSION
Through the climatic data obtained during the pre-harvest period, it was observed a variation in relative humidity (46 to 96%) and temperature (17.6 to 32.6 °C) (Figure 1A). The rainfall reached its maximum value of 39 mm (Figure 1A). During the harvest period, there was also a variation in relative air humidity (37 and 97%) and temperature (11.2 to 30.2 °C). The maximum rainfall during this period was 35.2 mm (Figure 1B).

Figure 1 Meteorological data from the municipality of Viçosa-MG during the pre-harvest period (02/23/2018 to 03/21/2018) (A) and harvest period (03/23/2018 to 05/10/2018) (B). Total daily rainfall (mm), daily maximum and minimum temperature (oC), and daily maximum and minimum relative humidity (RH %). Source: Meteorological Station 86824 located in Viçosa-MG. INMET (2018)
Assessing the climatic data is important, since the weather conditions directly interfere in the quality of soybean seeds, mainly when submitted to delayed harvest. In general, locations with high altitudes and low temperatures at night allow soybean seeds to remain in the field without suffering interference and quality reduction (Silva et al., 2016). However, even in the face of these and other favorable characteristics, harvest delay affects soybean seed quality. In this context, since the cultivars assessed have distinct maturity groups, plants reached the R8 stage on different dates and more than one harvest was necessary. Plants of cultivar ‘NS 5959’ reached the R8 stage on March 23, 2018, while for cultivars ‘BMX Potência’ and ‘TMG 1175’ this stage occurred on March 27 and April 3, 2018, and on April 2 and 10, 2018, respectively (Table 1). Thus, the seed moisture content was defined in each harvest performed (Table 1) and from these values, the means of each treatment were obtained (Figure 2).
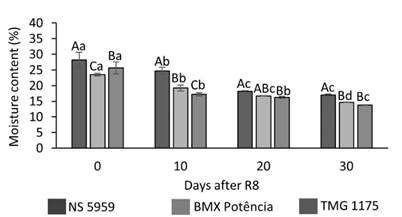
Figure 2 Moisture content of soybean seeds harvested at R8 and 10, 20, and 30 days after R8. Uppercase letters compare cultivars at each harvest time and lowercase letters compare harvest periods for each cultivar by Tukey test at 5% probability.
At all harvest times, there was a difference between the seed moisture content of the cultivars (Figure 2). In R8, the seed moisture content was lower for ‘BMX Potência’ (23.5%) and higher for ‘NS 5959’ (28.2%). Seeds of cultivar ‘NS 5959’, on the other hand, were exposed to rainfall on March 21, 2018 (Table 1 and Figure 1). Similar behavior was observed at R8 + 10 days, where the lowest moisture content was 17.2% (‘TMG 1175’) and the highest was 24.7% (‘NS 5959’), corresponding to the seeds of the cultivars less and more exposed to rainfall, respectively. At 20 days after R8, there was a decrease in the moisture content for ‘NS 5959’ (18.1%) and ‘BMX Potência’ (16.7%) and for ‘TMG 1175’ it remained similar to that obtained at R8 + 10 days. In general, lower values were obtained at 30 days after R8, ranging from 16.9 to 13.8% for cultivars ‘NS 5959’ and ‘TMG 1175’, respectively (Figure 2). Therefore, it is observed that, in general, there was a gradual decrease in the moisture content with the delay in harvesting the seeds of all cultivars evaluated, since the seeds harvested at the R8 + 20 and R8 + 30 stages were not exposed to rainfall in the pre-harvest phase (Table 1 and Figure 1). In general, the observed results of seed moisture content may be related to the fact that the cultivar ‘BMX Potência’ had the most harvests on dates when there was no rainfall (March 27 and April 3, 2018).
The analysis of variance of the data revealed significant interaction (p < 0.05) among harvesting periods and cultivars for the variables of germination, emergence, vigor index, and uniformity index (Figure 3).
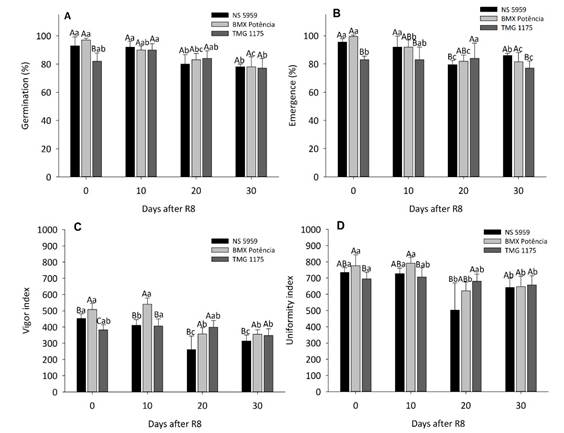
Figure 3 Germination (A), emergence (B), vigor index (C), and uniformity index (D) of soybean harvested at R8 and 10, 20, and 30 days after R8. Uppercase letters compare cultivars at each harvest time and lowercase letters compare harvest periods for each cultivar by Tukey test at 5% probability.
For germination, a statistical difference was observed only for seeds of different cultivars harvested at R8. At this stage, the cultivar ‘TMG 1175’ showed lower germination compared to ‘NS5959’ and ‘BMX Potência’, which did not differ statistically (Figure 3A). The most pronounced effect of the delayed harvest was observed in the germination of the cultivars ‘NS 5959’ and ‘BMX Potência’, which had significant reductions starting at R8 + 20 days. For ‘TMG 1175’ cultivar, there was a reduction in the germination percentage only at R8 + 30 days. Thus, it is important to consider that all seeds of the cultivars assessed, at all harvest times, showed germination above 80%, a value recommended for marketing soybeans seeds (Figure 3A). In the seedling emergence test, the cultivars ‘NS5959’ and ‘BMX Potência’ showed the highest averages compared to the cultivar ‘TMG 1175’ in the harvests performed at R8, R8 + 10, and R8 + 30 days. Similar to what occurred for the germination percentage, the most evident effect of the delayed harvest was identified in the emergence of seedlings of the cultivars ‘NS5959’ and ‘BMX Potência’, which from R8 + 20 days suffered significant reductions in comparison with periods in which the seeds remained less time in the field (R8 and R8 + 10 days) (Figure 3B). The effect of delayed harvest on the seedling performance of the different soybean cultivars can be observed by the results obtained for the vigor and uniformity indexes (Figures 3C and D). There was a statistical difference in the vigor index among cultivars in all harvesting periods, with the highest means observed for ‘BMX Potência’ at R8 and R8 + 10 days. However, in the other periods, the cultivar ‘NS 5959’ showed the most pronounced reductions. In general, the harvest delay in R8 + 20 and R8 + 30 days contributed to the reduction of the vigor index of seedlings of the ‘BMX Potência’ and ‘NS 5959’ cultivars. Regarding the cultivar ‘TMG 1175’, a more pronounced reduction in seedling vigor index was observed only at R8 + 30 days of harvest delay (Figure 3C).
A similar pattern to the vigor index was observed for the uniformity index, for which higher means were observed at R8 and R8+10 days for cultivar ‘BMX Potência’, which, however, did not differ statistically from ‘NS 5959’. At R8 + 20 days of delay in harvest, the cultivar ‘NS 5959’ showed the lowest average for the uniformity index among cultivars. At R8 + 30 days, there was no statistical difference between the cultivars assessed. As observed for the other variables analyzed, a reduction in the uniformity index was observed from R8 + 20 days for seedlings of the cultivars ‘BMX Potência’ and ‘NS 5959’. On the other hand, in the cultivar ‘TMG 1175’ a significant difference was observed only between harvests at full maturity (R8) and R8 + 30 days, being a less expressive effect when compared to the other cultivars (Figure 3D). In the periods of R8 + 20 and R8 + 30 days, a minor variation in physiological quality was observed among cultivars. However, the worst performance was observed for ‘NS 5959’, being the cultivar most sensitive to the adverse effects of delayed harvesting. The increased expression of genes related to lignin content (Castro et al., 2016), heat shock proteins, photosynthesis, and carbohydrate and lipid metabolism are noted as mechanisms of soybean seed tolerance to deterioration (Shu et al., 2020). These and other factors are inherent to the genotype and may explain the different behaviors among soybean cultivars subjected to harvest delay.
The physiological quality of seeds is a determining factor in plant growth (Idaryani et al., 2021), which was evident with the harvest delay seeds by 20 and 30 days, where seedling performance was affected through vigor and uniformity indices. Weerasekara et al. (2021) evaluated the effect of harvest delay on soybean seeds produced in different production environments and observed maximum seedling vigor index in harvests performed at the R8 stage. Furthermore, these authors observed a reduction in the vigor index with delays of five and 10 days in harvesting, pointing to climatic conditions and the number of days under late maturation as determinants for seed deterioration. The vigor index is based on the speed and uniformity of seedling development, and uniformity is based on the deviation of the length of each seedling evaluated (Pereira et al., 2020). This and similar indices are commonly used to assess the physiological quality of seeds (Medeiros and Pereira, 2018). In addition, indices such as vigor and uniformity are representative of initial growth (Silva et al., 2019). Therefore, the effects of deterioration caused by late harvesting in soybean seeds, by reducing the indices of vigor and uniformity, contribute to the formation of seed lots that generate seedlings with reduced and uniform growth. According to Oliveira et al. (2015), the higher initial growth of seedlings is of utmost importance in field conditions, because it allows for the greater acquisition of resources in the environment, which generates adult plants with greater photosynthetic capacity and greater productivity in the field.
The level of seed deterioration, besides being a result of environmental factors such as temperature and relative humidity, can be determined by the genotype (Pinheiro et al., 2021; Pinheiro et al., 2023). Therefore, among the analyzed cultivars, in general, the cultivar ‘TMG 1175’ showed the lower physiological quality of seeds at full maturity (R8) and at a close time (R8 + 10 days). This fact may reflect the higher rainfall incident between the different harvests performed during its development (Table 1). Rainfall during the final stage of seed maturation contributes to the reduction of physiological quality due to greater weathering damage, which is generated by the exposure of seeds to alternating cycles of high and low relative humidity in the environment (Castro et al., 2016; Pinheiro et al., 2021). In a previous study, Fialho et al. (2022) observed a reduction in germination and vigor of seeds of different soybean cultivars was found at R8 + 20 and R8 + 30 days. It was related to field deterioration due to delayed harvest.
As already mentioned, maximum seed quality is acquired at physiological maturity, at which time the supply of assimilates by the parent plant ceases and there are no longer significant increases in dry matter (Ellis, 2019). The interval between physiological maturity and harvest is seen as decisive for the physiological quality of seeds, as they are subject to adverse environmental conditions responsible for their deterioration (Fialho et al., 2022). Moreover, when it is not possible to harvest the seeds at the proper time, there is a reduction in physiological and sanitary quality (Goulart, 2018). As observed, delayed harvest by 20 and 30 days reduced the physiological quality of seeds of different soybean cultivars, affecting germination and seedling emergence. Reduced physiological quality of soybean seeds was also observed by Zuffo et al. (2020), who identified decreased germination percentage and seedling emergence from seeds harvested 14 days late after R8.
The seed health test showed a non-significant effect (p ≥ 0.05) of the cultivar x harvest time interaction for total fungal incidence (Figure 4). There was a gradual increase in total fungal incidence in seeds with the delay of harvest, with lower values at R8 and higher values at R8 + 20 and R8 + 30 days, which did not differ from each other. This increase was approximately 20 percentage points when comparing R8 + 30 days with R8 (control) (Figure 4A). On the other hand, no differences were observed in the total fungi incidence in the seeds of the different cultivars (Figure 4B).
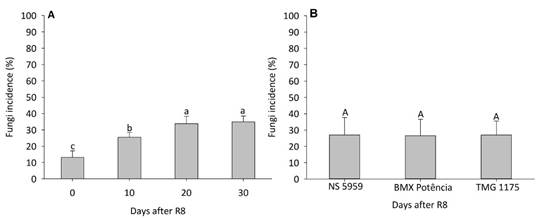
Figure 4 Incidence of fungi in soybean seeds harvested at R8 and 10, 20, and 30 days after R8. Uppercase letters compare the different cultivars and lowercase letters compare harvesting periods by Tukey test at 5% probability.
These observed results for seed sanitary reinforce the negative effect of soybean seeds remaining in the field after the R8 stage, having as a consequence the increased presence of pathogens in the seeds. It is important to reinforce the importance of detecting phytopathogenic fungi in soybean seeds, as they can cause a reduction in germination and initial seedling development, in addition to disseminating diseases in the field (Krzyzanowski et al., 2018).
The seeds of the three cultivars assessed were infected by different genera of pathogenic fungi, such as Aspergillus sp., Cercospora kikuchii, Phomopsis sp., and Cladosporium sp. However, the first three mentioned fungi occurred more frequently and had more relevance in this study. In general, the delay in harvesting after the R8 reproductive stage caused an increase in the incidence of the Phomopsis sp. pathogenic fungi in soybean seeds, especially with 20 and 30 days of delay in harvesting, and at 30 days this incidence was higher than 90% for all cultivars analyzed (Figure 5A).
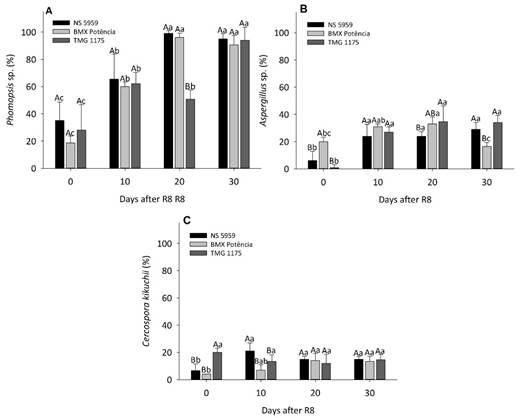
Figure 5 Incidence of the fungus Phomopsis sp. (A) Aspergillus sp. (B) and Cercospora kikuchii (C), in soybean seeds harvested at R8 and 10, 20, and 30 days after R8. Uppercase letters compare cultivars at each harvest time and lowercase letters compare harvesting periods for each cultivar by Tukey test at 5% probability
Regarding the incidence of Aspergillus sp. at R8 and R8 + 30 days, there was respectively higher and lower incidence of this fungus in the seeds of ‘BMX Potência’ cultivar when compared to the other cultivars. The presence of this fungus in the seeds of ‘NS 5959’ and ‘TMG 1175’ increased significantly with the delay of harvest starting at R8. At R8 + 30 days, there was a reduction of incidence in the seeds of the ‘BMX Potência’ cultivar (Figure 5B). Regarding Cercospora kikuchii (Figure 5C), there was a higher percentage of infected seeds of the cultivar ‘TMG 1175’ at R8 and ‘NS 5959’ at R8 + 10 days. In general, it can be stated that the seeds of all cultivars showed a higher incidence of this fungus at R8 + 20 and R8 + 30 days, although the values obtained were relatively low (between 12% and 15%). In general, there were no differences between cultivars regarding the occurrence of fungi as a function of harvest delay (Figures 4B and 5).
Among the main disease-causing pathogens in soybean crops that are transmitted by seed are Phomopsis sp. and Fusarium semitectum, which cause seed rot during the ripening and harvest stages; Cercospora kikuchii, which causes purple spot; and Aspergillus sp. which is responsible for seed rot in the soil (Goulart, 2018). Moreover, fungi of the Aspergillus sp. genus are among the main pathogens responsible for soybean seed deterioration during storage (Carvalho et al., 2021). Considering the different cultivars, these authors found that increased fungal incidence with delayed seed harvest after R8, being Phomopsis sp. the most frequent fungus, which corroborates the results obtained in this work (Figure 5A). According to Goulart (2018), fungi of the Phomopsis sp. genus are pathogens that infect the seed tegument and have their incidence increased in germination tests on paper roll under controlled conditions, which facilitate seedling contamination. Therefore, the reduction in seed germination and vigor with delayed harvest (Figure 3) may be directly related to the higher incidence of these fungi in the seeds (Figure 5). Aiming to reduce the detrimental effects of pathogens on seed quality after harvest, a common and important strategy is the chemical treatment of these seeds, which has been widely explored for soybean crops (Ferreira et al., 2019; Carvalho et al., 2020).
Multivariate principal component analysis (PCA) proved adequate to evaluate the relationship between the variables studied since the sum of the first two principal components (PC1 = 67% and PC2 = 17.4%) explained 84.4% of the total variation in the data (Figure 6).
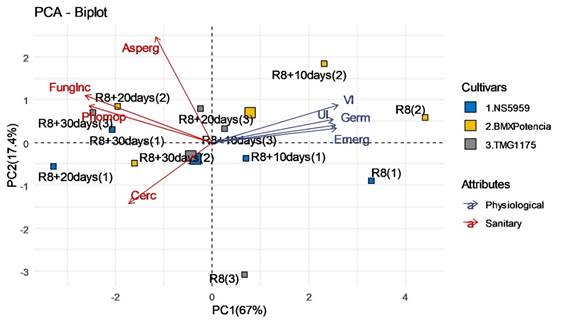
Figure 6 Biplot graph obtained by linear combination of variables related to physiological and health characteristics of soybean seeds harvested at R8 and at 10, 20 and 30 days after R8. Germination (G); Emergence (Emerg); Vigor index (VI); Uniformity index (UI); Fungi incidence (FungInc); Phomopsis (Phomop); Aspergillus (Asp); Cercospora (Cerc).
PCA allows for a more detailed analysis of the effect of harvest delay on soybean seed physiological quality. It is possible to observe that harvest treatment performed at R8 and R8 + 10 days, for the three cultivars evaluated, were positioned in the positive scores of PC1 and the same direction of the vectors of physiological quality (Germ, Emerg, VI, and UI). These results agree with those observed in the germination and vigor analysis, which attested to the higher physiological quality of seeds in harvests performed at and near physiological maturity (R8).
The treatments for harvests at R8 + 20 and R8 + 30 days, for all cultivars, were positioned in the negative scores of PC1 and opposite directions to the vectors of the physiological quality variables, showing less correlation and lower values for them. Furthermore, these treatments were concentrated near the vectors related to seed health (FungInc, Phomop, Asp, and Cerc). Thus, PCA allowed reinforcing that the treatments R8 + 20 and R8 + 30 days correlate with the lower physiological quality of seeds due to the greater fungal action in the deterioration that occurred in the field due to the delay in harvest and greater exposure to adverse effects in the field.
CONCLUSIONS
Delayed field harvest causes reduced germination and vigor of soybean seeds, especially when harvested at R8 + 20 and R8 + 30 days. Furthermore, it results in increased deterioration and incidence of phytopathogenic fungi in soybean seeds. In general, the cultivar ‘TMG 1175’ was more resistant to deterioration in the field caused by delayed harvesting.














