INTRODUCTION
A two-dose regimen of the Pfizer/BioNTech SARS-CoV-2 mRNA vaccine (BNT162b2) demonstrated 95% efficacy against Coronavirus disease 19 (Covid-19)1, but end-stage kidney disease patients were not represented in this population. Dialysis patients are susceptible to severe Covid-192,3 making them a priority group for vaccination around the world4. However, as these patients are known for their weak humoral responses to vaccines, such as hepatitis B5, concern has arisen on how well and for how long they might respond to BNT162b2. In the general population, the vaccine elicits high IgG and neutralizing antibodies responses within 15 days after the second dose, with titers peaking during the first month, but decaying afterwards6. This waning humoral response has led to booster doses being considered beyond the initially approved two-dose regimen7-9, especially given that breakthrough infection in BNT162b2-vaccinated persons has been correlated with neutralizing antibody titers10,11. However, a threshold titer that can predict breakthrough infection has not been defined and the duration of immunity is uncertain and probably heterogeneous. On the one hand, there are some groups of vaccinated infection-naïve individuals, including dialysis patients12, who have been shown to develop lower antibody levels, suggesting that antibody titers in these populations may decrease earlier than others. On the other hand, both vaccinated and unvaccinated people previously infected with SARS-CoV-2 elicit much stronger and durable antibody responses13,14. After two BNT162b2 doses, we evaluated the SARS-CoV-2 spike immunoglobin G (IgG-S1) antibody levels in previously infected peritoneal dialysis (PD) patients and compared them with infection naïve PD patients.
METHODS
In February 2021 we vaccinated 81 PD patients with the BNT162b2, twenty-one days apart. At close to four months post-vaccination, we tested these patients for seroconversion using the BioPlex 2200 SARSCoV-2 IgG Panel (BIO-RAD, Hercules, California, USA) and the SARSCoV- 2 IgG II Quant assay (Abbott Laboratories, Chicago, Illinois, USA).
The former is a multiplex immunoassay for the qualitative detection and semi-quantitative differentiation of IgG antibodies against the following targeted viral antigen: receptor binding domain of SARSCoV-2 spike protein, S1 domain of the SARS-CoV-2 spike protein, S2 domain of the SARS-CoV-2 spike protein and nucleocapsid (N) protein of SARS-CoV-2. A positivity cutoff is ≥ 10U/mL. The latter is a chemiluminescent microparticle immunoassay (CMIA). It is used for the semi-qualitative and quantitative determination of IgG antibodies to the receptor binding domain (RBD) of the S1 subunit of the spike protein of SARS-CoV-2 in human serum and plasma. A positivity cutoff is ≥ 50 AU/mL.
Infection naïve patients were considered those without a history of PCR confirmed Covid19 and negative BioPlex result for the anti-N IgG. Previously infected patients were those with a history of PCR confirmed Covid19. Due the lack of baseline serologies, patients without a history of Covid19, but with a positive anti-N IgG were excluded since we could not ascertain if the infection occurred before or after vaccination.
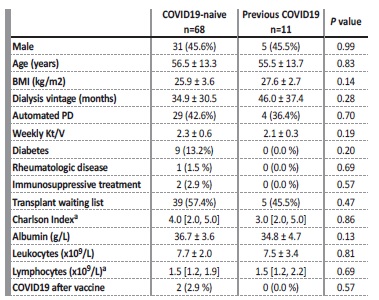
We analyzed both cohorts for gender, age, PD modality, dialysis vintage, weekly Kt/V, body mass index, previous immunosuppression, leucocytes, lymphocytes, albumin and comorbidities included in the Charlson Comorbidity Index. Continuous variables were expressed as mean ± standard deviation (SD) or median (range) if normally or nonnormally distributed, respectively. Categorical variables were represented by absolute and relative frequencies. Comparisons between infected and non-infected groups were performed using Chisquared test for categorical variables and Student’s t-test or Mann-Whitney U test for continuous variables if normally or non-normally distributed, respectively. We used multivariable linear regression to study the association between IgG-S1 levels and baseline characteristics. Statistical analysis was performed using IBM SPSS Statistics 25.
RESULTS
Eighty-one PD patients were given the BNT162b2, of which two were excluded because of a positive anti-N IgG without a history of Covid-19. Of the remaining 79, there were 68 infection naïve PD patients and 11 PD with previous Covid-19 (Table I). We found no statistically significant differences between groups. All patients were positive for IgG-S1 at a median of 112 days after the second dose.
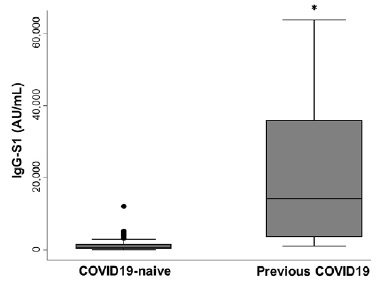
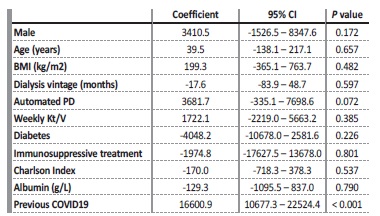
Median IgG-S1 titers in previously infected PD patients (14310 AU/mL; maximum 63639 AU/mL, minimum 1207,7 AU/mL) was significantly superior to median IgG-S1 in the infection naïve group (760,05 AU/mL; maximum 12130,7 AU/mL; minimum 50,4 AU/mL) (p < 0,001) (Figure 1). After accounting for covariates in a multivariate linear regression model, previous Covid19 was the only significant predictor of IgG-S1 levels (Table II). To date, we have identified two cases of mild Covid19 breakthrough infection in the infection-naïve group (IgGS1 of 368,7 AU/mL and 1541,6 AU/mL) and none in the previously infected group.
Table I Baseline characteristics by the state of previous COVID19 infection.
Values are presented as n (%) or mean ± standard deviation, unless otherwise noted.
BMI - body mass ndex; CI - comorbidity ndex; PD - peritoneal dialysis.
a Median [interquartile range]
Table II Multivariable linear regression for the association between IgG-S1 levels and baseline characteristics.
BMI - body mass index; CI - confidence interval
DISCUSSION
We aimed to determine one component of the immune response, the humoral response, of PD patients to BNT162b2 four months after a two-dose regimen. We searched for differences in the level of antibodies directed against the SARS-CoV-2 spike protein between infection naïve and previously infected PD patients. Despite a 100% seroconversion rate, we observed significantly higher SARS-CoV-2 antibody levels in previously infected compared with infection-naive individuals. We did not specifically measure neutralizing antibody titers, but they have been strongly correlated to IgG-S1 before6. Given that breakthrough infections have been correlated to lower neutralizing antibody titers10,11, the lower levels of IgG-S1 in the infection naïve group may result in a lower vaccine effectiveness and/or a shorter period of immune protection, whereas the higher levels of IgG-S1 amongst previously infected PD patients may imply just the opposite. In fact, studies in unvaccinated previously infected persons observed that both IgG and neutralizing antibody levels decrease only slightly at 8 to 10 months after the infection and that breakthrough infections are much less common13-15. Indeed, we experienced zero cases against two in the infection naïve group. This is in sharp contrast to antibody kinetics of vaccinated infection-naïve persons, who evidenced a significant decrease in antibody levels after 6 months6. Furthermore, previously infected individuals have been shown to exhibit higher antibody levels after only one dose of the BNT162b2 compared with infection-naive individuals after two doses16, which is a reason why, after February 2021, previously infected patients in Portugal were recommended only one dose of a vaccine, irrespective of its standard regimen being of one or two doses. Additional doses beyond the first in the previously infected population came to be regarded as booster doses.
Overall, we conclude that the humoral response to a two-dose regimen of the BNT162b2 in previously infected PD patients at four months post-vaccination is higher and predictably longer than in infection-naïve PD patients. This study is confirmatory for the PD population that, as in the general population, previously infected patients elicit a much stronger humoral response to the BNT162b2. It may be useful for discussions surrounding vaccination strategies of PD patients, highlighting the potential to withhold a third, BNT162b2 booster dose in previously infected PD patients already with a two-dose regimen, making additional doses available around the world. Limitations of this study include the small sample, the lack of neutralization antibody measurements and the lack of T-cell response studies.