CLINICAL PRESENTATION
We present a case of a 58-year-old male with chronic kidney disease of unknown origin (no prior biopsy), in hemodialysis since 1999. The patient had been subjected to a kidney transplant (KT) in 2003 with late graft loss after 12 years. Past medical history included hypertension, ischemic cardiomyopathy, peripheral arterial disease, and human immunodeficiency virus (HIV) infection diagnosed in 2011, treated with antiretroviral therapy (dolutegravir 50 mg and lamivudine 150 mg) since then.
In March 2022, the patient underwent a second deceased-donor kidney transplantation (men, 61 years, ischemic stroke) with two human leucocyte antigen (HLA) mismatches (one HLA class I and one HLA class II) with no donor-specific antibodies, virtual Panel of Reactive Antibodies (PRA) 0% and cold ischemia of 8 hours.
Routine preimplant biopsy was performed, light microscopy examination showed 5/29 glomeruli with sclerosis, interstitial fibrosis and tubular atrophy involving <20% of cortical and medium-sized arteries were well preserved. Banff score was: g0 i0 t0 v0 ah0 cg0 ci1 ct1 cv1 mm1 ptc0 ti0. As per routine, immunofluorescence was not performed in this sample.
The induction immunosuppression used was thymoglobulin (total dose 6 g/kg), tacrolimus, mycophenolate mofetil and prednisolone. Tacrolimus levels during early post-transplantation remain between 8-12 ng/mL.
The early post-transplant period was complicated by prolonged oligúria and need for hemodialysis until day 14 after the transplantation. There were no complications during the peri-operatory period, the surgery was uneventful, and the patient maintained hemodynamic stability.
Doppler ultrasound evaluation revealed allograft with 11.7 cm, maintained corticomedullary differentiation and reduced diastolic flow in the interlobar vessels (resistivity index 0.94-1). HLA antibody screening was negative at 48 hours and 10 days after transplantation.
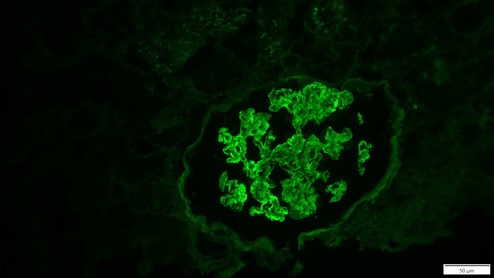
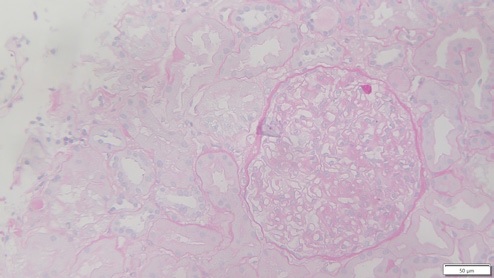
Thirteen days after KT, due to delayed graft function, the patient was submitted to a biopsy, which showed 26 glomeruli, one with global sclerosis, 3 with segmental sclerosis and some with ischemic changes (Fig. 1). Mild hyalinosis and intimal hyperplasia were also observed. Immunofluorescence revealed granular staining of glomerular capillary walls for IgG (++) and C4d (++) (Fig. 2). There was no significant glomerular staining for other immunoglobulins, complement fragments, kappa, lambda, albumin and fibrin. C4d was negative in the peritubular capillaries, and there was no other evidence of acute rejection. Banff score was: g0 i0 t0 v0 ah1 cg0 ci0 ct0 cv2 mm1 ptc0 ti0. Electron microscopy was not performed. The histological result of the biopsy was compatible with membranous nephropathy (MN) stage 1.
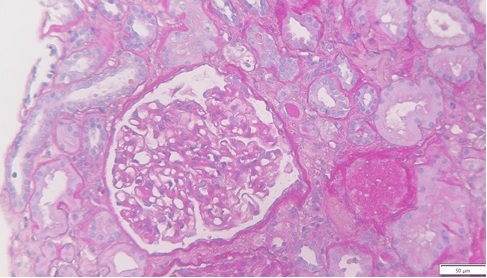
Figure 1: Periodic acid-Schiff (PAS) x25, recipient biopsy preformed 13 days post kidney transplant.
Urinary protein was unremarkable (protein/creatinine ratio <0.5 g/g) and did not increase during the clinical course. The patient was discharged 3 weeks after transplantation with serum creatinine of 4.22 mg/dL.
QUESTIONS
1. What is the most likely diagnosis, given the clinical history and presentation?
2. What is the final diagnosis considering the histopathological findings?
3. How should this patient be managed?
4. What is our patient’s prognosis?
ANSWERS
1. What is the most likely diagnosis, given the clinical history and presentation?
The patient presented with delayed graft function and required hemodialysis in the immediate post-transplant period. There was na important clinical and histological dissociation since the biopsy performed on the 13th day after transplantation did not show alterations that suggest ischemic acute tubular necrosis, which is the most common cause of delay in graft function.1 However, prerenal and postrenal causes were excluded and the evaluation by Doppler ultrasound showed an increase in resistivity index that may suggest intrarenal graft dysfunction.1 The patient had a favorable evolution in the first two weeks with recovery of diuresis and gradual improvement in graft function. MN is a common cause of nephrotic syndrome in adults and is one of the most frequent glomerular diseases found in the post-transplant period.2,3 MN is histologically characterized by uniform thickening of the glomerular basement membrane due to the presence of sub-epithelial immune deposits.3 These findings at this early time point, could represent a recurrent, de novo or donor-derived disease.
MN may recur after transplantation (30%-45% of cases), leading to proteinuria and increasing risk of graft failure.2,4 The recurrence of MN and these histological features can be observed early as 1-2 weeks after transplantation.2,5 The etiology of chronic kidney disease of the patient is unknown. Nonetheless, no evidence of recurrence of glomerular disease was observed in the 12 years after the first KT.
De novo MN is an uncommon complication of KT and usually occurs months or years after transplantation.4 However rare cases have been described to occur early after transplantation.3,4The disease may develop in a variety of circumstances, such as viral infections, allograft rejection, mechanical or ischemic injury, that predispose the allograft to the exposure of hidden antigens and thereby stimulate the production of circulating antibodies (typically of the IgG1 subclass), formation of immune complexes and the histological lesion of MN.4 KT from donors with MN have been previously reported and with it appearing to resolve with little or no impact on graft function.6,7
Preimplant biopsies can help the histological interpretation of subsequente graft biopsies. In this case, considering the early appearance of histological changes, the most likely diagnosis was MN from the donor allograft. However, more data on the donor and re-examination of the preimplantatory biopsy are needed.
2. What is the final diagnosis considering the histopathological findings?
The information concerning the donor in this case was limited, the terminal serum creatinine level was 0.97 mg/dL. The urinalysis reports, obtained prior to the patient’s death, had revealed proteinuria (albuminúria 1.7 g/day), without hematuria. The donor’s renal ultrasound showed kidneys with normal dimensions without alterations. Donor information such as prior history of hypertension, diabetes mellitus, monoclonal gammopathy or other was not available.
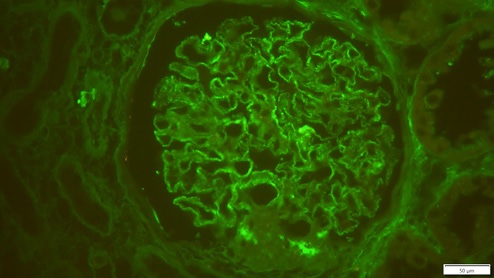
The donor preimplantation kidney biopsy was reexamined, with immunofluorescence revealing granular positivity in capillary walls for IgG (++), staining for other immunoglobulins, albumin, complement, kappa and lambda was negative, compatible with MN (Fig. 3).
IgG subclass staining showed a IgG1 dominance, and immunohistochemistry was negative for anti-phospholipase-2 receptor (PLA2R) antibodies. Pretransplant PLA2R antibodies of the donor’s serum were negative. PLA2R receptor antibodies were also negative. These data and IgG1 dominance favor the diagnosis of donor-derived secondary MN.3,8
3. How should this patient be managed?
The reported cases indicate that transplanted MN had no significant impact on graft function. Progressive washout of capillary wall electron dense deposits in serial biopsies is evident and IgG deposits can be detectable in capillary basement membrane months after transplantation.
Some reports demonstrated the persistence of these changes for more than 3 years after transplantation, but insignificant clinical manifestations.7,9,10
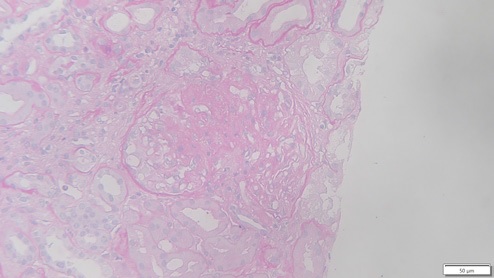
In this case, the allograft biopsy taken one month after transplantation displayed 11 glomeruli with two globally sclerosed, four with segmental sclerosis and one with ischemic changes. One had cellular crescent with fibrinoid necrosis, and the remainder showed thickening of the capillary walls (Figs. 4 and 5). These changes were interpreted in the context of donor glomerular disease. Banff score was: g0 i0 t0 v0 ah1 cg0 ci1 ct1 cv0 mm0 ptc0 ti1. Granular staining for IgG (++) and IgA (+) along the capillary wall were observed in the specimen.
Since pre-existing MN might show remission and studies with serial biopsies showed the resolution of immune complex deposits, protocol biopsies are important to assess the evolution of the disease, as well as monitoring proteinuria and graft function.
4. What is our patient’s prognosis?
Studies of kidney transplants with primary glomerular imune complex-mediated disease of the donor are uncommon and the most commonly reported is IgA nephropathy (9%-24% in donor biopsy specimens).9,11 To our knowledge, there are few reports of membranous nephropathy (MN) in kidney donor in the literature. In all reported cases, the immune complex deposits seemed to clear from the transplanted kidney tissue and appear to have no impact on kidney transplant outcome (graft function and proteinuria).10 A nonimmunogenic environment for MN plus immunosuppressive therapy can facilitate immune complex clearance, but prospective randomized trials are needed to confirm the reported results.12
In our case, protocol biopsy performed at six month showed improvement of histologic aspects of MN, classified as stage I. The kidney function is stable (serum creatinine 2 mg/dL) and proteinuria is 0.5 g/g. Further follow-up is required, but the prognosis seems good.
Data available on kidney donor pathology transmitted to the recipiente and their functional consequences for the graft is still missing, but successful outcomes have been reported.