Introduction
Women’s role in society has changed greatly throughout the years. They tend to be older when getting married or on their first intended pregnancy, although initiating active sexual life at a younger age. Therefore, contraception plays a major role in women’s emancipation. Although contraception has taken a great leap in the last decades, with the introduction of long-acting reversible methods and the creation of national and worldwide programs to make methods available and affordable to everyone, 40% of all pregnancies are still unintended (the rate being greater amongst adolescents)1. Therefore, it is important to know the different methods available, how they work, their contraindications, and which one is more suitable for each person.
Combined hormonal contraception (CHC) is a type of birth control that contains both estrogen and progestogen hormones. Approved for contraception in 1960, the estrogenic component made part of some contraceptive methods for cycle control, establishing the principle of combined contraception. CHC me-thods include oral pills, patches, and vaginal rings. The efficacy depends on the user compliance.
Contraception efficacy
CHC are a highly effective form of birth control when used correctly and consistently. The typical use failure rate of these methods is around 7%, which means that with typical use, about 7 out of 100 people who use CHC for one year will become pregnant. However, with perfect use (meaning using the method exactly as directed), the failure rate is less than 1%. It is important to note that no form of contraception is 100% effective, and the efficacy of CHC can be affected by several factors, such as missing doses, taking certain medications, and medical conditions that may interfere with the absorption or metabolism of the hormones2. This being said, it is understandable that oral routes of ministration are more prone to inadequate use, than non-oral routes (such as the vaginal ring or transdermal patch).
Mechanism of Action
The hypothalamus secretes Gonadotropin-releasing hormone (GnRH), which stimulates the pituitary gland to release Follicle-stimulating hormone (FSH) and Luteinizing hormone (LH). FSH stimulates the growth and development of follicles in the ovary, while LH surge triggers ovulation.
CHC contains natural or synthetic estrogen and progestin hormones, which work together suppressing the release of FSH and LH, respectively, as shown in Figure 1.
The estrogen component of CHC suppresses FSH secretion by negative feedback on the pituitary gland, resulting in the inhibition of follicular development.
The progestin component of CHC further suppresses LH surge by inhibiting the secretion of GnRH from the hypothalamus. Progestogen also thickens cervical mucus, making it more difficult for sperm to reach the egg.
Estrogen component
There are four types of estrogen available in CHC: ethinylestradiol (EE), estradiol valerate (VE2), estradiol (E2) and estetrol (E4).
EE is a synthetic form of estrogen with higher bio-disponibility which makes it one of the most commonly used in CHC3,4. It suffers hepatic regulation, influencing the synthesis of clothing factors, hepatic cell enzymes, serum enzymes and plasma proteins, potentially leading to a higher risk of cardiovascular events, such as deep vein thrombosis (DVT) and stroke. However, the risk is small and is outweighed by the benefits of preventing pregnancy in most women. The hepatic impact of EE is mainly related to its pharmacological potency (given by the ethinyl radical) and it’s independent of the route of administration and also of hepatic first pass. The new routes of administration (vaginal and transdermal) are therefore also not applicable to women with high vascular risk.
E2 and its ester VE2 are natural forms of estrogen less commonly used. Estradiol, the main natural estrogen produced by the ovary, has great affinity for proteins and is rapidly metabolized in the liver with consequent low bioavailability ≈ 5%5. CHC with E2 and VE2 have a lower risk of cardiovascular events compared to ethinyl estradiol3-7.
E4, a Native Estrogen with Selective Tissue action (NEST), is also a natural estrogen that is produced exclusively during pregnancy by the fetal liver and placenta. It has a unique mechanism of action compared to other estrogens8,9. It acts as a selective estrogen receptor modulator (SERM), which means it can selectively activate or block estrogen receptors in different tissues. E4 has a high affinity for the estrogen receptor alpha (ERα) and a low affinity for the estrogen receptor beta (ERβ). One of the unique benefits of estetrol is its minimal impact on blood clotting factors, which may make it a safer alternative to other estrogen therapies. Additionally, E4 has been shown to have a lower impact on liver function, which may be beneficial for women with liver disease. E4 has no active metabolites (end hepatic metabolization as sulfate and/or glucuronide conjugate) and no carcinogenic metabolites have been identified and has a low risk of drug interaction (is not metabolized by cytochrome P450).
Progestogen Component
Natural progesterone is not used for contraception, because the plasma concentrations of oral progesterone are very low (quick first pass metabolism).
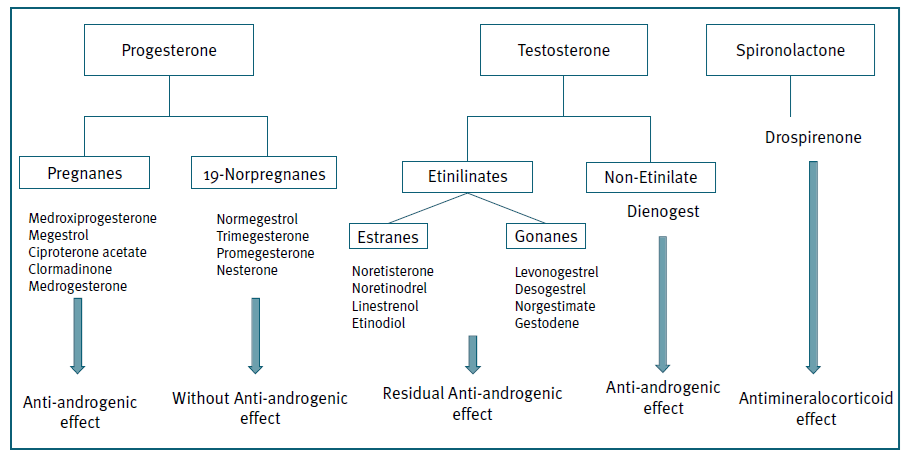
Progestogens are synthetic hormones used in CHC to prevent ovulation and thicken cervical mucus. There are several types of progestogens (Figure 2), divided in 4 groups (1 to 4 generation progestins), according to the timing of introduction as a contraceptive method. They can also be divided based on structural properties, which gives the contraceptive method its specificity, since they have different action in various receptors. The pivotal role of coregulator in the mechanism of action is shown as the intersection between the different receptor (AR = Androgen receptor; ER = Estrogen receptor; GR = Glucocorticoid receptor; MR = Mineralocorticoid receptor; PR = Progesterone receptor). The interactions between the different steroid receptors involved in the mechanism of action of hormonal contraceptives with activation of transcription of target genes causes induction of cellular therapeutic or adverse events. (Figure 3)
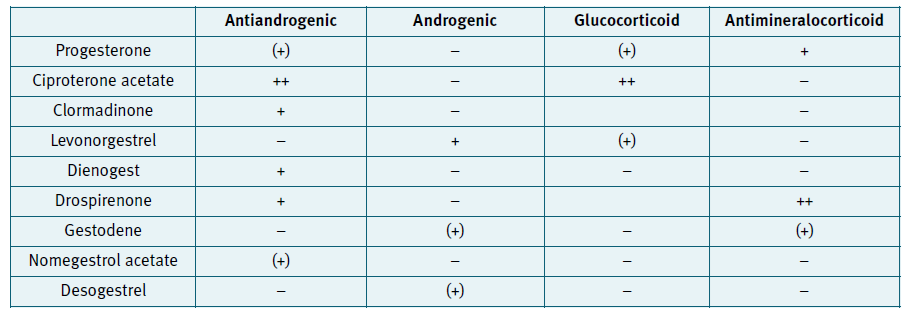
Figure 3 Progestins and their action in different progesterone receptors. Adaptated according to Schindler A. Maturitas 2008;61:171-180
First-generation pills contained a combination of higher EE dosage (35-50 µg), with progestogens with strong ability to bind to the PR (noretisterone or norethindrone acetate). Second generation oral contraceptives use progestogens such as levonorgestrel with a higher antigonadotrophic effect, allowing the reduction of EE to dosages of 25-30 µg10,11.
With the intent of lowering metabolic and vascular impact, third generation contraceptives were designed with progestogens (desogestrel, gestodene and norgestimate) with less androgenic side effects. They are also associated with excellent cycle control, reduced premenstrual bloating, dysmenorrhea, premenstrual mood fluctuations and breast tenderness. Third-generation pills contain 15-30 µg EE/tablet10.
Three associations of EE with a non-testosterone derived progestin (Ciproterona acetate, chlormadinone acetate and drospirenone) exist with some antiandrogenic properties. These compounds could be associated with a new ‘fourth-generation’ class, although the progestins used in these associations are from a different origin, which makes the class rather heterogeneous. Drospirenone is an analogue of the aldosterone antagonist - spironolactone, with both progestogenic and anti-androgenic activity, giving the method a diuretic effect.
The new progestins, because of their reduced androgenicity, do not adversely affect the cholesterol lipoprotein profile and have the potential to offer protection against cardiovascular disease, an important consideration as we enter an era of women using oral contraceptives for longer duration and later in life10.
Non oral routes
When we talk about non oral CHC we refer to the transdermal patch or the vaginal ring.
The transdermal patch contains 35 mcg EE+ 200 mcg Norelgestromin. Norelgestromin undergoes liver metabolism resulting in levonorgestrel a metabolite highly bound to SHBG, limiting its biologic impact12,13. The patch should be renewed every 7 days. In case the adhesive peels off for more than 24 hours, a new adhesive must be applied. A delay in starting a new patch cycle of up to 2 days poses no risk or affects the cycle, but any delay greater requires a new cycle start with backup contraception for 7 days. Activities such as exercise, bathing, swimming and use of the sauna/hydromassage bath don’t cause any changes in hormone levels.
The vaginal ring contains 15 μg EE + 120 μg etonogestrel daily. The urogenital and pelvic diaphragm muscles act as a sphincter of the vaginal introitus, and the almost horizontal position of the vagina minimizes the risk of expulsion14-17. Hormones absorbed by the vaginal mucosa enter the bloodstream directly, preventing hepatic first-pass metabolism. This allows usage of lower doses, preventing fluctuations in serum hormone levels and reducing potential adverse effects (e.g. irregular bleeding, nausea) 16,18-20. Routine use involves replacing the ring with a new one every four weeks to allow deprivation bleeding, but an easier method is to insert a new ring on the first day of each month. Continuous use of the vaginal ring is an option, as it contains enough hormones to prevent ovulation for at least five weeks. Breakthrough bleeding with continuous use is effectively managed by a 4-day hormone-free interval21. If removed during sexual intercourse, it should be reintroduced within 3 hours, to maintain its efficacy.
Compared to the patch, the systemic exposure to EE with the vaginal ring is 3.4 times lower, and it is 2.1 times lower than a 30 μg EE pill. The overall EE concentration in patch users is comparable to that of a 50 μg EE pill, which may be related to a heavier cardiovascular risk22.
The vaginal ring and transdermal patch have been shown to have an excellent profile in terms of general and gynecological tolerance and can be recommended for women who have difficulty in compliance to daily pill intake23,24.
Regimens
Any CHC can be started in three ways: on the first day of the menstrual cycle, on Sunday or immediately started on the same day if pregnancy is excluded (Quick-start). The quick-start option is more practical, but if started after 5 days of the onset of a new contraceptive free cycle, additional precaution should be used on the first week.
Classic 21 day regimen, with a 7-day Hormone Free Interval (HFI), was designed to mimic a physiologic cycle and is not based on medical necessity. 21/7 regimens can be associated with undesirable symptoms caused by fluctuating hormone levels during the HFI, like bloating, dysmenorrhea, headaches, and mood fluctuations.
The 24/4 regimens offer clinicians and patients the important advantage of reduced bleeding and the possible advantage of greater efficacy because of better compliance as well as a reduction in ovarian activity. In fact, these two outcomes are interrelated. Lower ovarian follicular activity leads to decreased fluctuation in endogenous estrogen levels, which in turn results in a more stable and inactive endometrium. Additionally, the regimen offers a clinical advantage of reduced risk of “escape” follicular activity if a patient accidentally starts a new pack one or two days later25-27.
The combination of E2 and NOMAC (nomegestrol acetate) in a monophasic 24/4 regimen has been shown to effectively suppress ovulation and provide good cycle control with minimal impact on non-reproductive systems28-30.
Extended and Continuous Regimens: Despite greater follicular activity with lower-dose oral contraceptives, most women still have effective prevention of ovulation. However, the emergence of follicular growth during the HFI, along with the awareness that lower-dose estrogen formulations can increase ovarian activity and cause bleeding, has led to the shortening of HFI. Continuous dosing of oral contraceptives, as well as the vaginal ring and patch, provides greater ovarian suppression, leading to a reduction in the potential for breakout and escape ovulations. Continuous dosing also simplifies the pill-taking schedule, improving compliance and lowering the failure rate. The return of ovulation and achievement of pregnancy are not delayed after discontinuation of continuous dosing.
Daily contraception can be used to treat conditions such as endometriosis, bleeding disorders, menstrual seizures, and menstrual migraine headaches, even to avoid bleeding in athletes and busy individuals. The idea that menstrual bleeding is a cleansing or detoxifying process is outdated in modern society, and bleeding may interfere with a woman’s sexual and social life, sport activities and work. So, regardless of age, many women prefer to have a menstrual period less often or not at all31,32.
The dynamic quadriphasic regimen with decreasing doses of VE2 and increasing doses of dienogest in a regimen of 26/2, has demonstrated high effectiveness and safety in real-life studies, as well as improvement in headaches and pelvic pain that occurs during the pill-free interval and less negative effects on sexual function30,33-41.
Drug Interactions
Due to its hepatic metabolism CHC may interact with some other drugs, altering its concentration and efficacy. It is important for the physician to be alert of these interactions: 42
Rifampicin is the only antibiotic proven to decrease serum levels of the CHC.
Anticonvulsants (phenitoine, carbamazepine barbiturates, primidone, topiramate, felbamate, or oxcarbazepine) may reduce the efficacy of CHC. However, it can be used if the patient understands the risks and cannot use other methods. In these cases, a formulation containing a minimum of 30 mcg of EE and progestins with a longer half-life (drospirenone, desogestrel, levonorgestrel, nomegestrol acetate) in a continuous 24/4 regimen should be preferred.
CHC increases lamotrigine clearance, resulting in a decrease in plasma lamotrigine concentrations by 45 to 60 percent. To avoid fluctuating levels it’s indicated using continuous regimens.
Griseofulvin (an antifungal) has been associated with contraceptive failure in several case reports.
HIV medications (namely Ritonavir associations, Efavirenz and Nevirapine) may decrease the effectiveness of CHC, so additional contraception should be used43.
Regarding the use of St. John’s wort, limited evidence suggests that coadministration with oral contraceptives may increase the risk of ovulation and decrease the effectiveness of CHC, by induction of cytochrome P450, and increase metabolism. This effect is dose dependent.
Non-contraceptive benefits
When talking about CHC, it should not be forgotten that in addition to preventing pregnancy, these contraceptive methods are associated with additional health benefits, such as reducing menstrual pain, regulating and reducing the intensity of menstrual bleeding. The antiandrogenic effect of CHCs allows their use as a valuable treatment option for acne and other signs of hyperandrogenism, especially those with antiandrogenic44.
The use of CHC is also associated with the reduction of the risk of developing certain cancers, such as ovarian and endometrial cancer. The longer a woman uses combined hormonal contraceptives the greater the reduction ovarian cancer risk is. However, this protective effect decreases after discontinuation48,49.
Other benefits of CHC include the preservation of bone density, decreasing the risk of ovarian cysts, and, if taken continuously, absence of menses, which is particularly important for women with anemia. Some of these benefits are more associated with the type of progestin used in the method, hence the importance of knowledge of the complaints and wishes of the patient when counseling about contraceptive methods.
Risks and adverse effects
CHC have some risks and adverse events that should be considered. The most common, and the major contributors for method discontinuation, include spotting, nausea, breast tenderness, bloating, but that are manageable with knowledge of the different hormone effects9,52,53.
The most serious adverse event is an increased risk of cardiovascular (CV) events, such as deep vein thrombosis (DVT), stroke (AVC), and myocardial infarction (MI). These risks are dose-dependent, which led to the development of formulations with doses between 15 and 35 mcg, currently used. The risk is small, but it is increased in women who smoke, have a history of cardiovascular disease, or have other risk factors for cardiovascular events. This risk is higher in the first year of use and has been related to the impact of EE on hepatic synthesis of procoagulant proteins.
Other side effects, such as hypertension, have also been related to the impact of EE on the hepatic syn-thesis of angiotensinogen.
The prothrombotic changes seem to be more important with third-generation methods and are associated with an increased risk of DVT. The pro-thrombotic effects of third-generation pills could be explained on the one hand by the increase in procoagulant effect, and on the other hand by the decrease in anticoagulant effects. Even though the amount of estrogen used is lower, third-generation progestogens could enhance the effects of estrogen on clotting factors, leading to a procoagulant effect45.
CHCs are contraindicated in women who have a history of idiopathic DVT. Women using CHCs should be aware of the symptoms of blood clots, such as sudden unexplained shortness of breath, chest pain, severe pain or swelling in the legs, rapid breathing or cough, and weakness or numbness of the face, arm or leg. and should seek medical advice immediately if they develop any of these signs and symptoms46.
There was a concern that the use of combined oral contraceptives (COC) could have a long-lasting effect on the development of atherosclerosis, which would be compounded by the natural aging process and potentially become evident later in life47. Arterial thrombosis typically occurs in women with damaged arteries as a result of diabetes, hypertension, smoking or obesity and not as a result of vasoconstriction. The impact of smoking on the risk of DVT is less than that on the risk of arterial thrombosis, but smoking, especially heavy smoking, may act synergistically with CHC46.
The interindividual variability in steroid metabolism is large. Therefore, it is not surprising that the same dose can cause side effects in one patient and not in another48.
The relationship between CHC and breast cancer in young women is controversial. The risk is not increased in women with benign breast disease or a family history of breast cancer. Breast cancer mortality does not vary significantly between women who have used CHC and those who have never used it. A 2013 meta--analysis showed that the incidence of breast and ovarian cancer in women carrying the BRCA1 to BRCA2 mutation was similar between CHC users and non-users48,49. Some data suggests that women who start taking oral contraceptives before the age of 20 have higher relative risks of breast cancer while taking the pill and up to 5 years after discontinuation, but this is a period when the incidence of breast cancer is very low. Therefore, the actual number of breast cancers that may be caused by early use of oral contraceptives is likely to be minimal. It seems reasonable to inform carriers of BRCA mutations that the use of oral contraceptives is likely to reduce the risk of ovarian cancer, but the effect on breast cancer risk is uncertain50. Some data associated oral contraceptive use for at least 5 years to double the risk of breast cancer before age 50 in BRCA2 carriers, but not in BRCA1 carriers51. Past CHC use may be associated with lower risk of later metastatic cancer and possibly lower risk of postmenopausal breast cancer47,50.
Breast tenderness and ovarian cysts in users of very-low-dose pills can be caused by diminished suppression of follicular activity in the ovary. Even though breast tenderness is an estrogen-related side effect, its treatment would be to further suppress the ovary by using a higher EE dose. In new users of CHC, it can be managed by lowering the EE dose or switching to a progestin with anti-mineralocorticoid activity (such as drospirenone). If it occurs in CHC users with 20 mcg EE, increasing the dose (to increase suppression of ovarian follicular activity) might work. If it occurs in usual users, it is important to exclude organic causes (hyperprolactinemia, thyroid disease) and consider a me-thod without estrogen54.
Irregular bleeding is a frequent reason for discontinuation contraception; however, there is no evidence that the onset of bleeding is associated with decreased efficacy55. Abnormal uterine bleeding (AUB) with CHC requires assessment of adherence, compliance, changes in intestinal transit, and drug interactions. Organic causes and chlamydial infection should also be excluded.
Headaches should be evaluated to exclude migraine with aura. In case of catamenial headache, VE2+Dienogest 26/2 regimen could be considered. Alternatively, switching to a 24/4 regimen with 15 mcg EE or a prolonged/continuous regimen, decreasing EE dose, switching to another progestin, or using a vaginal ring may be beneficial.
Sexual dysfunction associated with CHC is multifactorial. Before switching CHC, clinicians should assess whether changes in sexual function occurred after starting hormonal contraception, complaints of vaginal dryness or pain during intercourse, the couple’s definition of normal sexual function, and if any member of the couple is experiencing stress at work or in their private life. If CHC is still indicated, options include trying a more androgenic progestin such as levonogestrel, switching to VE2/Dienogest, or using a non-hormonal method56,57.
Contraindications
The World Health Organization has developed a Medical Eligibility Criteria for Contraceptive Use (WHO-MEC) system to help healthcare providers determine the safety and suitability of different contraceptive methods for individual patients, based on their medical history and other factors, categorizing contraceptive methods into four categories: 1 (no restrictions) to 4 (unacceptable risks). CHC must be avoided in category 3 (should not be used) and 4 (absolute contraindication) of the WHO-MEC.
In respect of CV risk, two contraindications remain: hypertension (possibly with the exception of those women under the age of 35 years with well-controlled blood pressure and with no complications) and the risk of deep vein thrombosis (DVT).
Breast cancer and other hormonal dependent tumors are also a contraindication for CHC58.
The association between estrogen therapy and the onset of acute pancreatitis has been established, especially with triglyceride levels >500 mg/dL. Therefore, a high triglyceride level should caution the use of estrogen treatment because of the risk of pancreatitis. COC should not be used in individuals with existing vascular disease59.
Smoking and CHC use is considered an absolute contraindication in women over 35 yo. In women younger than 35, heavy smoking (15 or more cigarettes per day) it is a relative contraindication58.
Systemic Lupus Erythematosus (SLE) patients can consider using progestin-only methods. However, in patients with stable or inactive disease, without renal involvement and high antiphospholipid antibodies, low-dose CHC can be considered. If hormone therapy is to be provided to patients with high-titer anticardiolipin antibodies, lupus anticoagulant, or previous thrombosis some form of chronic anticoagulation should be considered (such as low-dose aspirin) 60,61.
Considering that the incidence of obesity and subsequently the number of women bearing vascular as well as carcinologic risk factors is increasing, the search for adequate and efficient alternatives is a very important target for future research62. Different recent developments have targeted both goals of reducing compliance failure and impact on vascular risk.
Conclusion
Despite current changes in contraception methods, with the introduction of contraception with high efficacy and long lasting, CHC is still the elected method by most Portuguese women. The introduction of new estrogens and progestins with lower CVC risk and more favorable side effects have been the strong selling point of the newest CHC formulations.
The constant evolution wave on contraception, makes it indispensable for a constant update in knowledge in this field. This will allow better counseling and ensure the choice of the best method for everyone.