Introduction
Peripheral artery disease (PAD), consists of partial or complete obstruction of the arteries in the lower limbs and is one of the most common manifestations of atherosclerosis.1,2 It affects more than 202 million people worldwide.2 More than 20% of over 65-year-olds have PAD.2,3 The prevalence is higher in high-income countries than in low- and middle-income countries (7.4% versus 5.1%).4 The prevalence of PAD has increased by ≈45% globally (≈18% in high-income countries and ≈58% in low- and middle-income countries.4
The classic symptom of PAD is intermittent claudication, while chronic limb-threatening ischemia (CLTI) is the severe form of PAD.4 CLTI is defined by ischemic rest pain, tissue loss, or gangrene and the symptoms should be present for a minimum of 2 weeks.4 The prevalence of CLTI was 1.3% among individuals aged ≥40 years in a given year.4 This accounted for 11% of overall PAD. The prevalence of CLTI seemed largely constant through the years.4
Relatively little is known about factors associated with disease progression to CLTI and the reason for higher mortality in patients with established CLTI. We hypothesize that inflammation is a key role in evolution to CLTI and can justify in part the worst prognosis of CLTI.
The aim of this review is to evaluate the influence of inflammation on progression to CLTI. The second objective is to analyse if inflammation can contribute to a worst prognosis in patients with CLTI.
Methods
Data Sources and Search
The PubMed database was systematically searched at 28-29th of May and at 2nd-3rd of June 2022. The query was as follows:
(‘inflammation’ OR ‘cytokine’) AND (‘prognosis’ OR ‘death’ OR ‘mortality’) AND (‘CLTI’ OR ‘CLI’)
Inclusion and Exclusion Criteria
Studies were included in the current systematic review if they met the following criteria: (1) described the influence of inflammation on PAD evolution to CLTI (2) describing the consequences of inflammation on CLTI mortality (3) retrospective or prospective observational clinical studies, (4) performed in humans with PAD, (5) full-text available. Studies published in English, French, Spanish and Portuguese were eligible for inclusion.
Other studies were excluded for the following reasons: (1) papers analysing the consequences of inflammation on the success of revascularization (2) studies reported only as abstracts or with incomplete data, letters and nonclinical studies (3) systematic reviews or meta-analysis.
Results
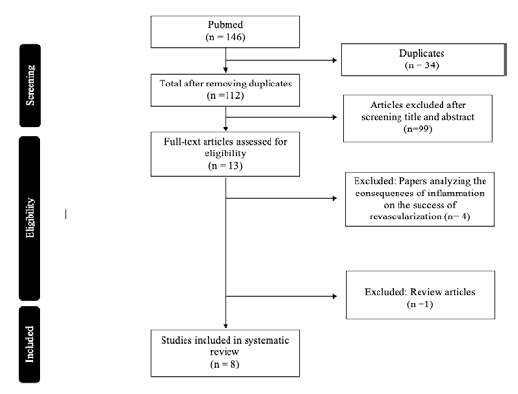
A total of 146 articles were found and, after removing duplicates, 112 articles were left (Figure 1). After reading all titles and abstracts, 13 full-text articles were read and a total of eight articles were selected.
The papers reviewed were published between 1999 and 2019, eight were from Europe and one from USA. A total of 1622 patients were included in this review.
Inflammation and Risk of Developing PAD
Atherosclerosis is one of the main causes of PAD and atherosclerosis is contemporarily seen as an inflammatory disease.3,5 Atherosclerosis consists of cholesterol deposition in the intima of large and medium size arteries, accompanied by a chronic inflammatory process.6 This is characterized by foci of macrophages and T-lymphocytes, proliferation and migration of smooth muscle cells, matrix formation and neovascularization.6 Inflammatory phenomena, at sites of atherosclerotic plaques, are major determinants of the progression of the disease.6 In particular, an increased infiltration of monocytes/macrophages in the plaques has been correlated with plaque fissuring and disruption, and the subsequent thrombotic phenomena.6
Inflammation is also important for the initiation and progression of PAD.1,7 There are several candidate inflammatory triggers, including the traditional risk factors, such as advanced age, nicotine abuse, diabetes mellitus, hypercholesterolemia, and arterial hypertension.1,2,8 They exert a proatherogenic role, at least in part, through an inflammatory mechanism.1
Cigarette smoking and diabetes mellitus which are the strongest predictors of developing PAD, promote oxidative stress and enhances inflammatory pathways.1 Hypertension, which affects about 80% of patients with PAD is also related to inflammation.1 Dyslipidemia may activate inflammatory functions by modifying the oxidation of low-density lipoproteins and of very low-density lipoproteins.1
These traditional cardiovascular risk factors, though useful in population studies - fail to explain the variable nature of disease progression between individuals.5 Previous papers have shown that some patients with PAD have a combination of non-traditional risk factors which are characterized by a persistent systemic inflammatory response.8 This risk factors include a history of infectious disease, autoimmune disease, or a genetic risk with polymorphisms in genes associated with inflammation.8 Tumor necrosis factor- (TNF-) α, neopterin, interleukin-6 (IL-6) and other cytokines are inflammatory mediators reported to be involved in atherogenesis.5,8 IL-6 has been shown to be associated with the development of PAD in the Edinburgh Artery Study.8 It was also found that an elevated IL-6 levels in patients with severe claudication.8 In the Rotterdam study, C-reactive-protein (CRP) and IL-6 were inversely related to ABI after adjustment for age, smoking status, body mass index, and diabetes mellitus.1
A case-control study demonstrated that IL-6, Intercellular Adhesion Molecule 1 (ICAM-1), E-Selectin, Monocyte-Chemoattractant Protein-1 (MCP-1), Matrix-Metalloproteinase-1 (MMP-1), and Matrix-Metalloproteinase-3 (MMP-3) gene polymorphisms were independently associated with the presence and severity of PAD in a hospitalized Italian population.10 This study also shows that synergistic effects between these pro-inflammatory polymorphisms determine the genetic profiles that are associated with different levels of risk for PAD and CLTI, depending on the number of high-risk genotypes concomitantly carried by a given individual.10
CLTI and Inflammation
Relatively little is known about factors associated with disease progression and major clinical events in established CLTI.8 However, it is well known that patients with CLTI frequently have a severe, aggressive, and generalized atherosclerosis.3,11 CLTI is associated with markedly elevated levels of several cytokines, chemokines and growth factors.11The wide range of circulating cytokines associated with CLTI resembles more a malignant inflammatory disease.11
Further evidence of a link between inflammation and PAD severity is the finding that levels of inflammatory markers parallel the staging of the Fontaine classification. In a study comparing 19 patients at Fontaine stage I with 19 patients with claudication, levels of high-sensitivity CRP and IL-6 were significantly higher in stage II patients than in stage I patients, and the latter group did not differ from the control group.1
In another study 132 patients with claudication were compared with, 30 patients with CLTI. It was concluded that high-sensitivity CRP levels in claudicants were intermediate between those of a control group and those of patients with CLTI. CRP progressively increased in PAD patients with worsening clinical Fontaine stage.1
In a study of 14 different cytokines in 101 CLTI patients compared to 37 healthy controls Teraa et al. reported that circulating levels of Vascular Endothelial Growth-Factor (VEGF), Stromal cell-Derived Factor-1α (SDF-1α), Stem Cell Factor (SCF), Granulocyte-Colony Stimulating Factor (G-CSF), IL-6, IL-8 and Interferon γ-induced protein 10 (IP-10) were significantly elevated in patients with CLTI when compared to the healthy controls.3
Patients with CLTI had significantly higher IL-6 levels compared to patients with claudication and IL-6 levels were inversely correlated with ABI.8
In multivariable linear regression modelling taking into account the baseline differences between intermittent claudication and CLTI groups, CLTI was independently associated with elevated levels of a large number of cytokines.3
Another research work published in 2016 analysed the levels of 48 circulating cytokines in 226 patients with PAD. It was verified that CLTI. was independently associated with elevated levels of a large number of cytokines.3
The current findings indicate that CLTI is associated with a circulating cytokine profile, which resembles a serious medical inflammatory condition.3 These data can help to explain the malignant cardiovascular behaviour and poor outcome associated inpatients with CLTI.3
Inflammation and poor long-term prognosis of PAD
Although PAD is not directly life threatening, affected patients have a higher risk of morbidity and mortality due to generalized atherosclerosis.5 Both symptomatic and asymptomatic PAD are associated with an increase in mortality, but most importantly cardiovascular mortality has been shown to be highest among patients with lower limb atherosclerosis when compared to atherosclerosis of other vascular beds.1
Patients with CLTI face a significantly worse outcome than those with a milder disease state.3 Generally, the 3-year survival rate in PAD patient cohorts has been around 70%, but most deaths are a result of CLTI. The mortality rate of patients with CLTI can be up to four times higher when compared to patients with intermittent claudication.3 For some specific patient populations with CLTI the 3-year survival rate can be as low as 33-35%.3 This fact can be explained by the hypothesis that a pronounced intravascular inflammatory process of the affected limb, caused by the presence of multiple “active” plaques, could be associated with more severe coronary and carotid atherosclerosis by a distinct pathophysiological mechanism.1
Several studies show that circulating markers of systemic inflammation are associated with future cardiovascular events. Gremmel’s et al proved that inflammatory biomarkers, such IL-6, IL-8 and IP-10 were predictors of major events in CLTI.8 In another paper, the inflammatory mediators IL-6, TNF-α, neopterin, and CRP were associated with 1-year mortality in subjects with CLTI.9
The 1-year mortality in patients with CLTI is influenced by features of inflammation such as leukocyte count and fibrinogen.9 Flex et al demonstrated that long-term prognosis of PAD patients is significantly associated with elevated plasma levels of inflammatory markers, such as CRP and fibrinogen.10
In 51 patients who underwent lower-limb revascularization, elevated CRP levels were associated with an increased risk of myocardial infarction after adjustment for the Eagle score index and previous coronary artery disease at 2 years of follow-up.1 CRP was significantly associated with both all-cause and cardiovascular mortality among patients who died during the 2 years after CRP assessment.1
In another study of a well-defined population with intermittent claudication, increased plasma levels of soluble vascular cell adhesion molecule-1 were associated with a 4-fold increased cardiovascular risk.1
In conclusion, increased inflammation may help explain why the prevalence of clinically manifested coronary artery disease in PAD is much higher than the prevalence of PAD in coronary artery disease and why the coexistence of PAD in coronary artery disease patients portends more severe coronary and carotid atherosclerosis.1
Clinical Implications
Patients with PAD and particularly with CLTI with elevated inflammatory parameters have a more aggressive atherosclerosis and should be immediately treated.12 The medical therapy should be improved with the prescription of anti-inflammatory agents such as angiotensin converting enzyme inhibitor (ACEi) and statins. A future avenue of research may be the use of immunomodulatory drugs such as those used in patients with auto-immune diseases in patients with “inflammatory PAD”. A timely intervention is also defensible because there is a partially reversion in inflammatory markers after revascularization.1,11,13,4 A timely revascularization may not only be limb saving but also avoid systemic inflammation and consequently global deterioration.
Conclusions
Inflammation is a key element in PAD progression and CLTI prognosis. Timely anti-inflammatory treatment and revascularization could decrease the deleterious consequence of inflammation in PAD patients, and should be liberally used.
Funding
This work was developed under the scope of project NORTE-01-0145-FEDER- 000013, supported by the Northern Portugal Regional Operational Programme (NORTE 2020) under the Portugal Partnership Agreement, through the European Regional Development Fund (FEDER), and by National funds, through the Foundation for Science and Technology (FCT) - project UIDB/50026/2020 and UIDP/50026/2020.
This work is also supported by the Portuguese Society of Angiology and Vascular Surgery (SPACV).