Introduction
Endovascular treatment with fenestrated endoprostheses (FEVAR) is a minimally invasive therapy for complex aortic diseases with suitable mid and long-term results.1 However, this treatment is not available in emergency settings. The parallel grafting technique can be an excellent alternative to prevent death, avoiding the higher morbidity and mortality of open surgical repair.2,3 We present a case of a zone 6 aortic pseudoaneurysm treated with a parallel grafting technique. Informed consent was obtained from the patient for the use of clinical data.
Case report
A 44-year-old male, born in Guinea-Bissau, presents to the emergency department with epigastric pain radiating to the back. The patient had a history of large vessel vasculitis secondary to Bechet’s disease (HLA B51 allele positivity).
Approximately ten years before, the patient was admitted to the hospital with left flank pain associated with fever. A computed tomography angiography (CTA) demonstrated the presence of a pseudoaneurysm involving the left common and external iliac arteries and another aortic paraceliac pseudoaneurysm. Also, left iliofemoral deep vein thrombosis secondary to compression was observed. The patient was treated with an aneurysmectomy and an in-line bypass from the left common iliac artery to the left common femoral artery using an 8mm ringed ePTFE graft. Due to the high perioperative hemorrhagic risk precluding full anticoagulation, a vena cava filter was put in place. Infection was excluded and a positron emission tomography (PET) scan revealed fludeoxyglucose uptake at the level of these vessels. A diagnosis of large-vessel vasculitis was assumed, and immunosuppression was started.
One first attempt to embolize the paraceliac pseudoaneurysm was made but was unsuccessful. Thus, the pseudoaneurysm was excluded with a 28mm thoracic endoprosthesis deployed juxta-superior mesenteric artery (SMA) ostium for an adequate landing zone, occluding the ostium of the celiac trunk. No visceral ischemic symptoms appear due to collateralization.
The patient maintained close surveillance with an annual CTA. Two years before the current episode, a pseudoaneurysm at the aortic graft distal terminus was observed. Considering the young age and low surgical risk, treatment by open surgery was proposed, but the patient refused. So, endovascular therapy with a custom-made fenestrated endoprosthesis was planned.
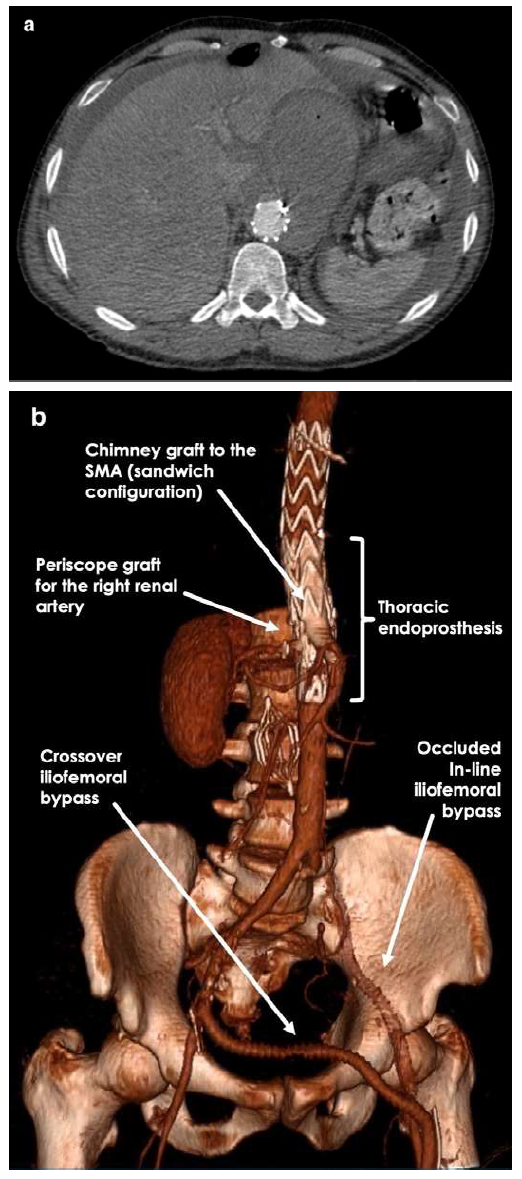
However, while waiting for the custom-made graft, he went to the emergency department complaining of epigastric pain with radiation to the back. An urgent CTA demonstrated significant growth of the pseudoaneurysm with approximately 10 cm in greatest diameter (Figure 1).
The patient did not meet the MAGIC criteria, excluding graft infection. Emergent treatment of the zone 6 pseudoaneurysm was accomplished with a parallel grafting technique. We surgical approach the right external iliac artery (EIA), the left femoral bifurcation, and the left brachial artery with the patient under general anesthesia. Through the left femoral access, we cannulated the right renal artery. We tried to catheterize the left renal artery from the same access, but it was impossible due to acute angulation. The SMA was cannulated through left brachial artery access, and a right brachial approach was made to left renal artery catheterization. A periscope graft for the right renal artery (7x100mm) and chimney grafts for the SMA (10x100mm) and left renal artery (6x100mm) were put in place. All stent-grafts used were Viabahn® self-expandable covered stents (SECS) (W. L. Gore and Associates Inc, Flagstaff, Arizona, USA). Through the right EIA, we advance a 31x100mm GORE® TAG® thoracic endoprosthesis (W. L. Gore and Associates Inc, Flagstaff, Arizona, USA) overlapping 5cm of the previous graft.
Partial deployment of the thoracic graft was followed by the release of the bridging stent-grafts. Migration of the left renal stent-graft to the aortic bifurcation occurred during deployment because it was not adequately positioned inside the renal artery, having been removed with an endovascular snare system. The aortic graft excluded the left kidney. No post-deployment ballooning has been made. Completion angiography showed total exclusion of the pseudoaneurysm. A crossed right to left iliofemoral bypass using an 8mm ringed ePTFE graft was necessary due to a dissection of the left iliac axis at the end of the procedure. Postoperative CTA confirmed the exclusion of the pseudoaneurysm and permeable vascular stent-grafts (Figure 2). The patient maintained a favorable evolution having completed only prophylactic antibiotic therapy during hospitalization. He was discharged 14 days after the procedure.
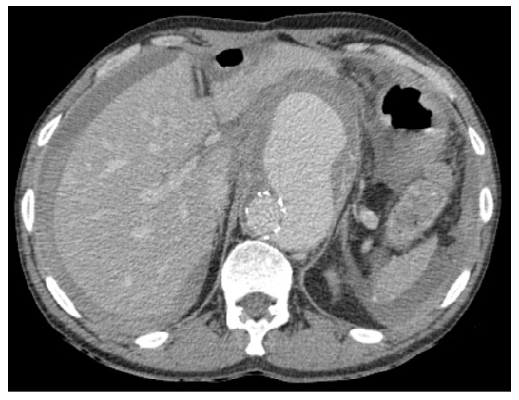
Figure 1 Pre-operative computed tomography angiography. Axial view computed tomography angiography revealed a zone 6 (Ishimaru zone) pseudoaneurysm with contained ruptured at the immediate terminus of the previous implanted thoracic graft.
Discussion
Urgent management of symptomatic/ruptured thoracoabdominal aneurysms or pseudoaneurysms involving visceral branches is challenging. Open surgery is a possible solution, however, not all centers have the necessary experience with this type of aortic reconstruction, and few patients have the physiological status to handle such invasive intervention.
Off-the-shelf branched endografts can correct extensive and complex aortic aneurysms in urgent settings. These devices have anatomical limitations, and long-term results are not well established.4 Furthermore, its availability is limited to some centers. This option would not be viable in our case since the patient already had a thoracic endoprosthesis occluding the celiac artery. Similarly, physician-modified endografts seem effective and safe in treating complex aortic aneurysms with a reported technical success of 91.4% and a major adverse event rate of 12.8%, but long-term results are lacking.5 Bearing in mind the nonavailability of a specific laser fiber for in-situ fenestration, the higher risk of target vessel endoleak, and lack of experience, we did not use this technique.
The parallel grafting technique presents a viable and cost-effective solution adaptable to different aneurysm morphologies that can be used in emergencies.6 Safety and efficacy of this technique have been reported in extensive studies. The PROTAGORAS study and PERICLES registry have shown high technical success and good medium-term outcomes with a low reintervention rate.7,8 It is important to note that most of the patients included in these studies had only one or two target vessels parallel grafts. The use of three or more parallel grafts is often not recommended.
In our case, some anatomical specifications must be considered. The patient had a previous thoracic aortic graft with occlusion of the celiac artery. Using a chimney graft in the SMA in a sandwich-like configuration allowed us to take advantage of the previously placed endograft and ensure an adequate proximal sealing zone, reducing the risk of endoleaks.9 We needed to cover the ostium of the renal arteries for a good distal landing zone, so we decided to use periscope grafts for both renal arteries. Unfortunately left renal artery graft migrated, and the left kidney was excluded. Appropriate sizing and attention to detail during stent deployment are essential to avoid this complication.
Aortic graft oversizing of about 20% was planned for optimal apposition due to the intended use of three bridging stents. Also, we opted for SECS as bridging stents that could have advantages in more pronounced angulation and offer long-term resistance to deformation forces. Controversy still exists since balloon-expandable covered stents have higher radial force with a lower tendency of compression but possibly more increased risk of gutter-related type 1 endoleaks. The risk of compression and infolding could be reduced with internal reinforcement with a balloon-expandable stent.10
In conclusion, open surgical aortic reconstruction or endovascular treatment with custom-made grafts are the preferred treatments for complex aneurysms of the thoracoabdominal aorta but could not be available in emergency settings. Our case highlights that the parallel grafting technique should be encouraged in life-threatening scenarios as an “off-the-shelf” solution for complex aortic repair, even in a previously reconstructed aorta.