Background
Extracranial carotid aneurysms (ECCA) are extremely rare, representing up to 4% of peripheral artery aneurysms and up to 2% of all carotid procedures.1-3 As axial imaging methods are becoming more commonly utilized, the incidental detection of ECCA is also on the rise. When symptomatic, they frequently manifest as a cervical pulsatile mass with compressive symptoms or cerebral thromboembolic events.1,2,4 However, the rupture risk is low.4 While atherosclerotic disease, prior surgery, or neck trauma are the most frequently reported etiologies, other causes include infection, fibromuscular dysplasia, connective tissue disorders, previous radiation exposure, and congenital defects.1-2 Although open surgery has been the traditional approach, endovascular repair is increasingly considered a potential alternative. However, due to the rarity of this condition, there are no clear guidelines or defined treatment algorithms. This paper aims to shed light on treatment selection and procedure details by presenting a small case series describing our center's experience in treating extracranial carotid aneurysms using diferente techniques, between 2020 and 2022. The ECCA were classified according to Attigah et al. classification.5
Case reports
Four cases of patients with aneurysms involving the extracranial internal carotid aneurysms are presented.
Characteristics of patients are summarized in the Table.
Table. Patient characteristics and procedural details
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Age | 60 | 61 | 36 | 77 |
| Sex | Male | Female | Male | Female |
| Clinical presentation | Asymptomatic | Asymptomatic | Asymptomatic | Nerve compression symptoms |
| Aneurysm classification | Type III aneurysm of the carotid bifurcation | Type I aneurysm of the proximal ICA | Type II aneurysm of the ICA | Type I aneurysm of the distal ICA |
| Maximum diameter | 20mm | 17mm | 19mm | 27mm |
| Treatment choice | CCA-ICA bypass (6mm ePTFE) + reimplantation of ECA | Aneurysmectomy and TT anastomosis | Aneurysmectomy and interposition (vein graft) | Exclusion with a stent-graft (transcervical approach) |
| Follow-up | 2 years (Patent; Uneventfully) | 1 year (Patent; Uneventfully) | 8 months (Patent; Uneventfully) | 6 months (Postoperative stent-graft occlusion) |
AHT: Arterial Hypertension; CCA: Common Carotid artery; ICA: Internal Carotid Artery; ePTFE: Expanded polytetrafluoroethylene; TT: termino-terminal
Case 1
A 60-year-old male with a history of hypertension, dyslipidemia, and atrial fibrillation presented with an asymptomatic 20mm type III aneurysm of the carotid bifurcation (Figure 1A). No aneurysms were found in other vascular territories. He underwent arterial reconstruction with an interposition bypass using a 6mm ePTFE graft with reimplantation of the external carotid artery (ECA) on the graft (Figure 1B). Intraoperative cerebral perfusion monitoring was performed with near-infrared spectroscopy (INVOS™), without significant drop after carotid clamping, thus shunt use was not considered. The prosthetic conduit was chosen to minimize the risk of future aneurysmal degeneration. The patient initiated single antiplatelet therapy with acetylsalicylic acid in the immediate postoperative period, to maintain lifelong. There were no complications after two years of follow-up. Histological findings were compatible with atherosclerotic etiology.
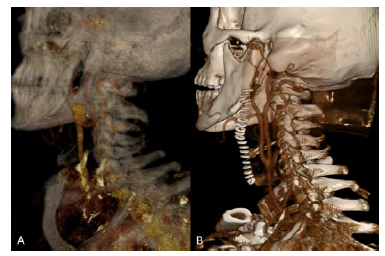
Figure 1 Pre and post-operative computed tomography angiography. A - 3D reconstruction showing a type III carotid bulb aneurysm with involvement of the origin of internal and external carotid arteries; B - Post-operative 3D reconstruction.
Case 2
61-year-old female with a history of hypertension, dyslipidemia, and plunging goiter presents with an asymptomatic 17mm type I saccular aneurysm of the internal carotid artery (Figure 2). No aneurysms were found in other vascular territories. Underlying auto-immune diseases were excluded, and imagological findings did not suggest fibromuscular dysplasia. Aneurysmectomy and direct reconstruction through an end-to-end anastomosis were possible due to artery redundancy. Intraoperative cerebral perfusion monitoring was performed with INVOS™ without significant drop after carotid clamping, thus shunt placement was waived. Single antiplatelet therapy with acetylsalicylic acid was initiated in the immediate postoperative period and maintained lifelong. The patient reported complaints of facial hypoesthesia after surgery, which improved after the 12th month of follow-up. Anatomopathological findings showed an artery wall with extensive hyalinization and areas of myxoid degeneration of inconclusive etiology.
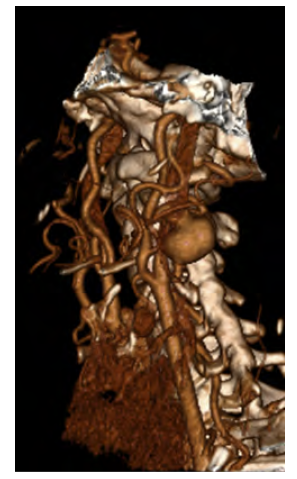
Figure 2 Pre-operative computed tomography angiography. 3D reconstruction showing a type I aneurysm of the internal carotid artery.
Case 3
36-year-old male with a history of Cushing's syndrome due to a secretory adenoma of the pituitary gland presented with a 19mm type II fusiform aneurysm of the internal carotid artery (Figures 3A and B). Contralateral internal carotid and basilar ectasia were also found. After pituitary gland tumor resection, he underwent arterial reconstruction with an interposition using a saphenous vein graft (Figure 3C). Intraoperative cerebral perfusion monitoring was performed with INVOS™ without significant drop after carotid clamping, and the use of a shunt was unnecessary. The autologous conduit was preferred, given the need for chronic corticotherapy to prevent the risk of graft infection. The patient was kept under single antiplatelet therapy with acetylsalicylic acid to be continued lifelong. No complications were observed by the 8th month of follow-up. Histological findings showed atherosclerotic plaque in the initial stage with an area of arterial wall dissection but with no findings of fibromuscular dysplasia.
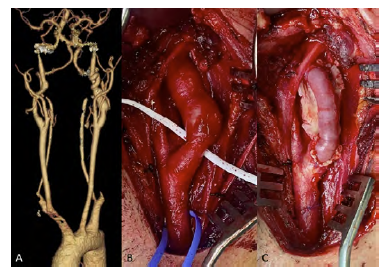
Figure 3 Pre-operative computed tomography angiography and Intra-operative images. A - 3D reconstruction showing a type II aneurysm of the internal carotid artery; B - carotid bifurcation and type II fusiform aneurysm of the internal carotid artery prior to reconstruction; C - reconstruction with a saphenous vein interposition.
Case 4
77-year-old female with a history of non-Hodgkin lymphoma treated with chemotherapy presented with facial pain compatible with trigeminal neuralgia. Angio-CT scan showed a 27mm type I aneurysm of the petrous segment of the internal carotid artery (ICA), confirmed by angiography (Figure 4A). No other aneurysms were found, and potential etiologies such as vasculitis were excluded.
Endovascular aneurysm exclusion using a self-expandable covered stent was performed through a cervical carotid surgical approach (Figure 4B) to avoid aortic arch manipulation. ICA catheterization was achieved with a hydrophilic guidewire supported by a Berenstein catheter but with wire coiling within the aneurysm. A 4mm balloon was inflated in the distal ICA to anchor a catheter, allowing the exchange to a Terumo® Stiff guidewire to support the deployment of the stentgraft Viabahn 5x100. Stent occlusion and common carotid artery dissection, presumably related to the puncture site were observed in the postoperative angio-CT (Figure 4C) but without neurological symptoms and with resolution of complaints associated with neurological compression. Dual antiplatelet regimen, initiated in the immediate postoperative period, was suspended after stent occlusion, and the patient was kept under single antiplatelet with acetylsalicylic acid.
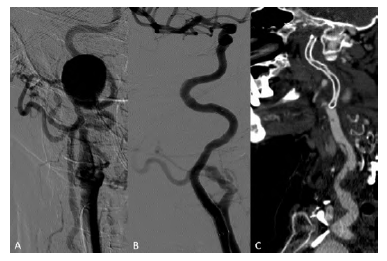
Figure 4 Digital subtraction angiography and post-operative computed tomography angiography of a type I aneurysm of the internal carotid artery. A - Pre-operative angiography; B - Completion angiography after aneurysm exclusion with a stentgraft; C - Computed tomography angiography (sagittal view) showing a small dissection of the proximal internal carotid artery and stent occlusion.
Discussion
Atherosclerosis with subsequent aneurysmatic degeneration is the most common cause of extracranial carotid aneurysms. This series reflects the previous premise with all but one patient with known cardiovascular risk factors; patient 3, despite his age, suffered from long-term non-controlled hypertension associated with the Cushing Syndrome, probably contributing to wall dissection, as anatomopathological findings corroborated. Also contralateral ICA and basilar artery ectasia were observed; in fact, the coexistence of cerebral aneurysms and pituitary adenomas were reported in 7,4% of cases in previous series.6 The mechanism and relationship between Cushing’s disease and aneurysmatic degeneration is not entirely understood7 but non-controlled hypertension may contribute to the pathophysiology. Patient 4 was the only patient without previous history of cardiovascular risk factors or any medical history other than non-Hodgkin lymphoma cured several years before. Radiotherapy has been associated with an increased risk of aneurysmatic degeneration;2,8 however, we found no relationship described in the literature between previous chemotherapy or the lymphoma itself and this condition.
There are no specific guidelines to support the decision-making for the optimal approach, and the best timing for ECCA intervention remains unclear. Due to the high risk of embolization, aneurysm repair is generally recommended in aneurysms greater than 2 cm, with mural thrombus, or associated with neurological or compressive symptoms.9 Open surgery yields favorable early and long-term results.2-10 However, it is associated with considerable perioperative morbidity with up to 66% risk of peripheral nerve damage, 11% risk of perioperative stroke, and 7% risk of perioperative death.1-3,5,10-16 In our series, three of the four patients were successfully treated by open surgery; only one patient reported symptoms of peripheral nerve damage with facial hypoesthesia, which significantly improved after the first year of follow-up. Patients 1 and 3 were treated with interposition bypass surgery; except for mycotic aneurysms, there are no recommendations regarding the best conduit. In patient 1, we used a 6mm PTFE graft, given the need for carotid bifurcation reconstruction with ECA reimplantation, minimizing the risk of aneurysmatic degeneration of a venous bypass; for patient 3, an autologous conduit was preferred due to the demand for long-term steroid therapy with a higher risk of infection. According to the Attigah classification, distal type I and proximal type V aneurysms may be particularly suitable for endovascular repair.1,5,17However, open surgery with aneurismectomy and primary end-to-end anastomosis was the preferred approach for patient 2, given the high tortuosity and redundancy of the artery.
Endovascular techniques are emerging as an alternative to open repair, minimizing perioperative morbidity, especially related to nerve damage and wound complications, but with a theoretically higher risk of thrombus embolization and worst long-term patency. Nonetheless, existing data on endovascular treatment’s short- and medium-term outcomes are comparable to surgery. In Li Z. et al.‘s systematic review,3 including 224 patients treated with a stent-graft with a median follow-up of 15 months, patency was achieved in 93,5% of patients. More recently, Hoffman M. et al. published another systematic review including 959 ECCA1; 750 patients were treated with open surgery with a 9% rate of cranial nerve injury, 4% rate of perioperative stroke, and a 2% rate of perioperative death; in the endovascular group (n=85) there were no cranial nerve injuries reported nor perioperative strokes or deaths, with one stent stenosis by the 12th months of follow-up. Despite these promising results, not all patients are suitable for endovascular repair. In our series only patient 4 was eligible for endovascular treatment, given the distal aneurysm localization. Nonetheless, stent occlusion was verified on a control angio-CT scan on the 3rd postoperative day, which may have been precipitated by the pre-stent dissection. The results of Song Xue et al. underscore the high technical failure rate observed in patients with severe artery tortuosity, which may also explain the propensity for dissection and the early stent occlusion observed in our patient.18
In conclusion, this paper highlights the importance of pathology classification for categorizing the main morphological subtypes to find the best treatment algorithm. Thus, considering each patient's specific characteristics, an individualized approach may lead to improved outcomes and reduced complication rates. Further research is warranted to refine our understanding of the factors influencing endovascular outcomes and optimize patient selection criteria for each procedure. However, large scale guidelines maybe precluded by the rarity of this entity.















