Case Description
A 43-year-old female was referred for suspected lymphoproliferative disease. Two years earlier, in 2020, the patient had complaints of progressive dyspnea on minor efforts and coughing episodes, so a diagnosis of asthma was made in 2020 (respiratory function tests were compatible with severe obstruction). The patient did not smoke and had no other relevant medical history.
Due to recurrent episodes of hemoptysis and thoracic oppression, she went to the emergency department where a chest CT was performed, showing an intraluminal tracheal mass of soft-tissue attenuation, inseparable from the posterior wall of the trachea and centered at the level of the middle third of the trachea. The hypothesis of lymphoproliferative disease was first proposed.
A magnetic resonance imaging (MRI) was performed. It showed an oblong expansive retro-tracheal mass in the superior mediastinum (fig. 1), with slightly lobulated and well-defined contours, hypointense on T1-weighted images and hyperintense on T2- weighted images. On T1-weighted fat-saturated images, this lesion showed homogeneous enhancement after intravenous gadolinium administration. The mass showed invasion of the posterior wall of the trachea along almost its entire length with luminal obliteration greater than 50% and invasion of the esophageal wall at its most superior aspect. The MRI signal and morphologic characteristics of the lesion were suggestive of a tracheal neoplasm. Given the imaging findings and clinical data (43-year-old non-smoking female), the hypothesis of adenoid cystic carcinoma was proposed.
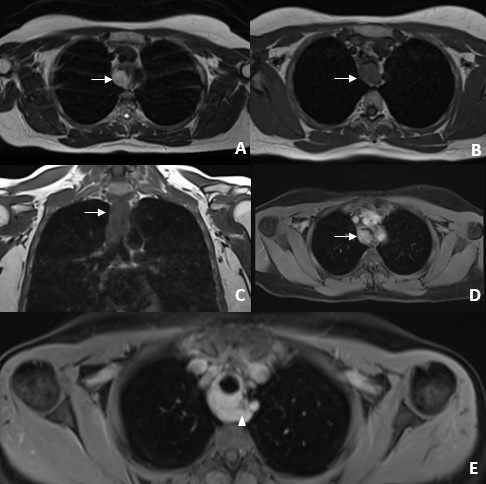
Figure 1: Chest MRI axial T2-weighted image (A) shows a hyperintense oblong expansive retrotracheal mass with well delimited and slightly lobulated contours. Axial and coronal T1-weighted images (B and C) show a slightly hypointense lesion causing luminal obliteration greater than 50% (arrows). On T1-weighted images with fat saturation after contrast administration (D and E), this lesion demonstrated homogeneous contrast uptake. At the most superior aspect of the lesion, we see direct invasion of the esophagus (arrowhead).
A positron emission tomography - computed tomography (PET-CT) was performed (fig. 2) which showed a high metabolic lesion posterior to the trachea. No other focus of high metabolic disease was found.
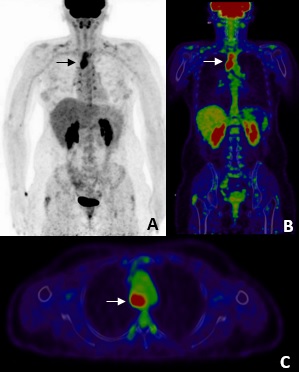
Figure 2: 18F-FDG PET-CT with MIP (A) and fusion images in coronal and axial planes (B and C) showed hyperfixation of 18F-FDG (SUVmax = 10.77) in the mediastinal mass (arrows). No other relevant hypermetabolic changes were found.
Ultrasound endoscopy with cytohistological sampling was performed (fig. 3). Immediately after the procedure, the patient had an episode of moderate hemoptysis and cardiorespiratory arrest, with reversal after advanced life support. In this context, she performed bronchoscopy for hemorrhagic control, which documented active hemorrhage in a wide-based vascularized mass (fig. 4). Given the risk of re-bleeding and the tracheal obstruction, it was decided to place a tracheobronchial stent (Novatech® GSSTM Y-STENT with 14/10/10 mm of outer diameters and lengths after adjustments of 9 cm for tracheal branch, 3 cm for left bronchial branch and 1.5 cm for right bronchial branch).
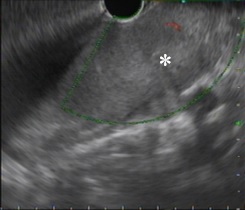
Figure 3: Ultrasound endoscopic image demonstrating hypoechoic vascularized solid lesion (asterisks). Invasion of the esophageal wall was also noted.
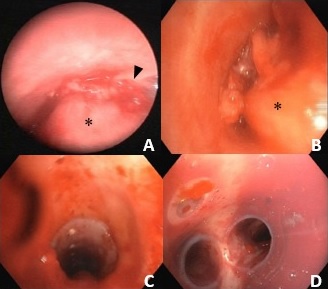
Figure 4: Bronchoscopy shows a wide-based obstructing lesion in the posterior wall of the trachea (asterisks) with active bleeding (arrowhead) in images A and B. In the images C and D at the end of the procedure, a well-placed tracheobronchial silicone Y-STENT can be seen repermeabilizing the airway.
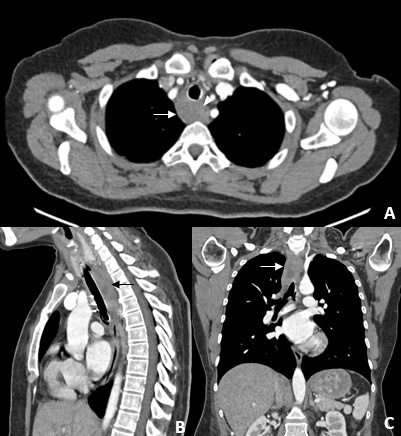
Figure 5: Multiplanar images of chest CT (A, B and C) showed the tracheobronchial stent, with proximal end inferior to the cricoid cartilage and two distal ends in the main bronchi, with all segments permeable and no signs pneumomediastinum or pneumothorax. An infiltrative mass is observed circumferentially involving the trachea, with infiltration of the retro-tracheal mediastinal fat (arrows). The lesion does not show a cleavage plane with the right anterolateral wall of the proximal and middle thirds of the esophagus (arrowhead), which is compatible with suspected esophageal invasion documented in previous exams. No lymphadenopathy or lesions suspected of secondary involvement were identified.
A chest computed tomography (CT) was performed due to chest pain and dyspnea maintenance, and it showed the tracheobronchial stent correctly placed, without associated complications (fig 5).
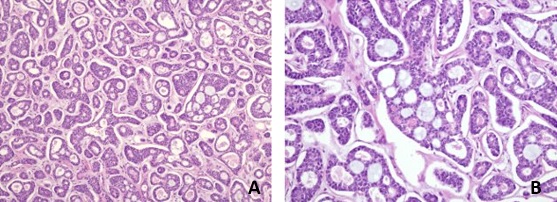
In the collected sample, bronchial mucosa was observed with extensive involvement by epithelial phenotype neoplasia, whose cytological and histological characteristics were compatible with salivary gland type neoplasia (ACC) (fig 6). The sample also showed a rearrangement involving the MYB gene, supporting the diagnosis.1
Although there is still no consensus on a staging system, according to some proposals described in the literature based on TNM tagging system, the patient was staged as cT4N0M0 given the signs of invasion of the adjacent esophageal wall.2,3
Considering the clinical staging and the longitudinal extension of about 8 cm (greater than 50% of the length of the trachea), the neoplasm was considered unresectable, so carbon-ion radiotherapy was proposed in Heidelberg, Germany.
In the follow-up, 8 months after radiotherapy, a marked reduction in tumor volume was observed. The tracheal stent was removed without complications and no suspicious lesions were identified at bronchoscopy. On serial CT and MRI imaging studies, the patient maintained stability of a slight upper and posterior peri-tracheal tissue thickening.
Discussion
Primary malignant tracheal tumors are rare.4 In adults, the majority are squamous cell carcinomas (SCC), the second most frequent being ACC, approximately 30%.5 Tracheal ACC is a low-grade, slow-growing malignant tumor that originates in the submucosal glands, occurring more frequently in the distal trachea.6 Unlike SCC, ACC is associated with younger adults, with some female predominance, and there is no correlation with smoking.4
The most frequent clinical picture is related to airway obstruction, including cough, wheezing, dyspnea and, more rarely, hemoptysis. Given the nonspecific clinical presentation, patients are often initially misdiagnosed with other more frequent pathologies such as asthma,7 as it happened in this clinical case.
CT is considered the best imaging approach, allowing localization, characterization and determination of its luminal and extra-luminal components, as well as the degree of tracheal obstruction, as demonstrated in the present clinical case.8 Lesions are typically soft tissue masses with more frequently slight or moderate uptake of iodinated contrast product, with polypoid or broad-based morphology with plaque-like or circumferential involvement and may have intra-luminal, intra-mural growth and/or extra-tracheal extension. Longitudinal extension is usually the longest axis of the lesion, being a characteristic finding of ACC and crucial to the therapeutic decision.5,9The tumor may have well-defined, lobulated or irregular margins and the existence of calcifications is rare. ACC can arise in the trachea, main bronchi, lobar bronchi, and more rarely in segmental bronchi. Perineural invasion is also characteristic.9 About 10% of patients have lymphatic involvement or metastases at diagnosis, most of them in the lungs.3 Magnetic resonance imaging can also help to delineate tumor extension.10
Bronchoscopy is frequently used to collect histological samples, thus allowing confirmation of the diagnosis. In our case, sample collection was performed through echo-endoscopy, which was additionally useful to corroborate previously suspected esophageal invasion.
There is no established staging system, but some groups have proposed systems based on the TNM staging.2,3
Segmental surgical resection with end-to-end anastomosis is the best therapeutic strategy in localized disease. Longitudinal extension greater than 50% of the length of the trachea precludes the surgical approach, markedly reducing the prognosis of our patient.3,11
The existence of positive margins after surgery is frequent, given the submucosal and perineural infiltrative behavior, justifying the frequent use of adjuvant radiotherapy.5 Isolated radiotherapy is used in patients who are not surgical candidates.11,12
Carbon ion radiation therapy is a recent and promising therapy option, being able to increase target doses while minimizing radiation exposure to adjacent organs at risk.13
This case highlights the difficulty at early diagnosis from clinic, respiratory function tests and imaging studies. The symptoms overlap with other diseases at an early stage such as asthma and the imaging characteristics can be easily mistaken with other most common lesions, thus delays in diagnosis and use of inadequate complementary diagnostic methods can occur. This context causes not only an increase in costs, but also a diagnostic delay that can make the lesion unresectable, with a very significant clinical impact on the prognosis of these patients.
In conclusion, radiologists and other medical professionals should be aware of tracheal neoplasms, including ACC. These lesions must be routinely and actively searched on imaging and should be considered in the differential diagnosis of mediastinal masses. ACC has some suggestive imaging features that should raise the diagnostic suspicion. Imaging is key for early detection, staging and resectability, which are critical to the therapeutic approach.