Introduction
Helicobacter pylori are spiral-shaped, microaerophilic, and Gram-negative bacilli [1]. H. pylori infection is ranked as the most common chronic bacterial infection in humans, with an estimated worldwide prevalence of 44.3% (50.8% in developing countries vs. 34.7% in developed countries) [2]. This bacterial pathogen colonizes the human stomach and via the inflammation caused to the gastric mucosa it is linked to the development of acute and chronic gastritis, peptic ulcer disease, gastric adenocarcinoma, and gastric mucosa-associated lymphoid tissue lymphoma [3, 4]. Studies have shown that treating an active H. pylori infection can alleviate its associated complications. Therefore, current guidelines recommend that all patients with an active H. pylori infection should receive treatment [5, 6]. The H. pylori eradication strategy is based on a combination therapy of antibiotics and acid suppressants, i.e., proton pump inhibitors (PPIs). The choice of an antibiotic depends on several factors, namely local resistance profiles, prior antibiotic therapy, history of allergy to specific antibiotics, patient age, renal and hepatic function and comorbid conditions, drug costs, adverse effects, and ease of administration [7, 8]. Due to the growing increase in antibiotic resistance, the efficacy of antimicrobials in eradicating H. pylori infection has significantly decreased. Therefore, finding alternatives or adjuvant therapies to antibiotic treatment may aid in improving the current eradication strategies [9].
Statins, or hydroxymethylglutaryl (HMG)-CoA reductase inhibitors, are employed to improve the lipid profile and are widely recommended in the management of patients diagnosed with hypercholesterolemia and mixed hyperlipidemia [10]. In addition to their cholesterol-lowering effects, statins are known to possess anti-inflammatory, immunomodulatory, and antimicrobial effects via direct antibacterial activity and synergistic activity with antibiotics [11-13]. Several studies have pointed out that statins can decrease the burden of H. pylori in macrophages via stimulation of autophagy [14]. Thus, the addition of a statin to standard treatment regimens, as adjuvant therapy, has been investigated in order to increase the H. pylori eradication rate.
In this systematic review and meta-analysis of data derived from previously conducted studies, our objective was to investigate the beneficial and potential effects of adding statins as adjuvant therapy to the standard treatments on the eradication of H. pylori infection.
Methods
Search Strategy and Study Selection
The present meta-analysis examines the influence of the addition of a statin to the current treatment strategy with regards to its effect on the H. pylori eradication rate. To conduct this research, we employed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) methodology. To prevent any bias, the research, selection of studies, qualitative evaluation, and data extraction were performed independently by 3 investigators and the agreement between the search results was reviewed by a fourth researcher.
We searched the available literature that focused on the additive effect of statins on the H. pylori eradication rate. The PubMed/MEDLINE, Web of Science and Scopus databases were searched for relevant papers published in English until September 15, 2020 by using the following keywords and word combinations: [(statin) OR (atorvastatin) OR (fluvastatin) OR (lovastatin) OR (pitavastatin) OR (pravastatin) OR (rosuvastatin) OR (simvastatin) OR (“hydroxymethylglutaryl-CoA Reductase Inhibitor”) OR (“HMG-CoA Reductase Inhibitor”) OR (“antilipemic agent”) OR (“lipid-lowering agent”) OR (“LDL-lowering agent”)] AND [(“Helicobacter pylori”) OR (H. pylori)] AND [(eradication) OR (treatment) OR (elimination)] in the Title/Abstract/Keywords fields.
Quality Assessment
The quality of the included studies was evaluated using the CONSORT checklist. A study with a quality score of 40-70% of the maximum score was rated as having a moderate quality. The studies receiving scores higher and lower than this score were considered as having high and low quality, respectively. The risk of bias was also assessed for all the papers in terms of random sequence generation, allocation concealment, participants and personnel blinding, blinding of outcome assessment, and outcome reporting according to the Cochrane standard form.
Selection Criteria and Data Extraction
As the main inclusion criteria, we selected the randomized controlled trial and observational studies which evaluated and reported the effect of adding a statin to the current treatment strategy for the H. pylori eradication rate. All authors independently evaluated the titles and abstracts and, based on the exclusion criteria, excluded reviews, case reports, editorials, guidelines, letters to the editor, conference abstracts, animal studies, irrelevant or duplicate studies, and articles with no clear methodology or results. The full-text of the relevant articles was assessed.
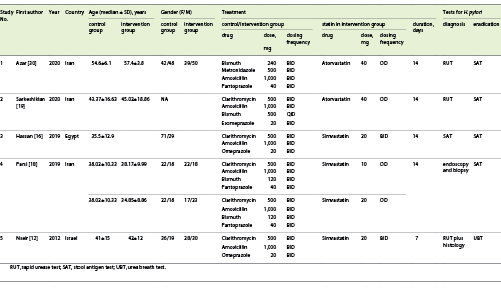
The following information was extracted from each study: study (first author, year of study, year published, and country); patients (sample size, age, and gender); test used for H. pylori infection diagnosis and eradication (rapid urease test, histology, bacterial culture, serology, stool antigen, urea breath test); treatment regimens for H. pylori and details regarding the statin used as adjuvant therapy (drug dose, dosing frequency, and duration), H. pylori eradication rate.
Publication Bias
The publication bias was analyzed using the Egger’s linear regression test and the funnel graph.
Definitions
Patients diagnosed with H. pylori infection had a positive result of any of the following tests: endoscopic-based tests (rapid urease test, histology, bacterial culture) or non-invasive tests (serology, stool antigen, urea breath test). The H. pylori treatment regimens consisted of a combination therapy of antibiotics and acid suppressants, namely PPIs. The eradication was assessed via the same diagnostic tests except serology at least 4 weeks after treatment initiation. A positive result of any of the aforementioned tests indicated treatment failure.
Statistical Analysis
Data management, transformation of effect sizes, and calculation of the pooled relative risk (RR) were performed using Stata software version 14. For each study, the RR was calculated as the measure of the association/effect size. The RR definition in our study was a ratio of the probability of H. pylori eradication occurring in the exposed group (the group who received standard treatment plus statins) versus the probability of H. pylori eradication occurring in the non-exposed group (the group who received standard treatment). The pooled RR and its corresponding 95% confidence interval (CI) were an estimation used in the meta-analysis to judge the effect of statin addition on the H. pylori eradication rate.
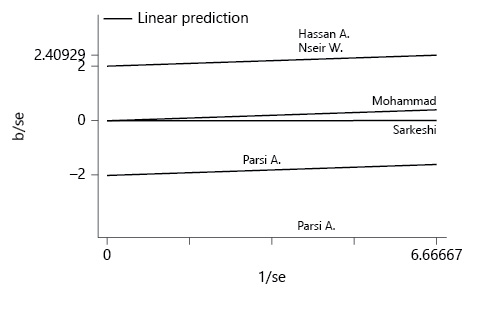
In addition to conducting a meta-analysis of the data using a fixed-effects model, since considerable heterogeneity was expected, all the analyses were also performed using a random effects model to estimate the pooled RR using the maximum likelihood estimation method. To check for heterogeneity between studies, both the I 2 test of heterogeneity and the χ2 test of heterogeneity (p < 0.1) were used. The I 2 was calculated to describe the percentages of total variation across studies caused by heterogeneity. A 0% value meant no heterogeneity, and higher values represented an increase in heterogeneity. Generally, heterogeneity is ranked as 25% (low), 50% (moderate), and 75% (high) [15]. The Galbraith plot was also used to detect the source of heterogeneity.
The results presented are based on the 5 included studies. To examine the possibility of publication bias, we first used the Egger method, which quantifies the bias captured by the funnel plot by regressing the standard normal deviation on precision defined as the inverse of the standard error. The presence of publication bias was further inspected using a funnel plot which plots a measure of the study size (precision or standard error) as a function of the effect size. Generally, the visual inspection of a funnel plot provides an indication of publication bias when smaller and larger studies are non-symmetrically distributed across the combined effect size [16], however it is performed only in the meta-analyses which include a sufficient number of studies (more than 5).
Results
Search Results
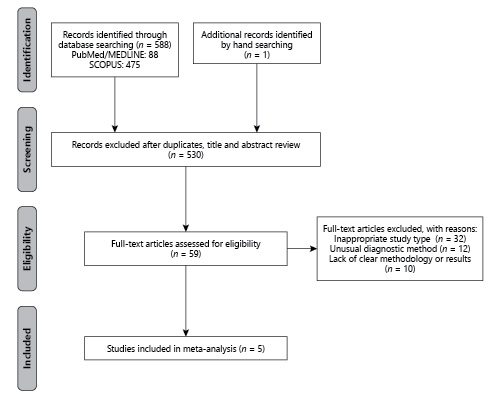
A total of 589 records were identified in the initial search of the databases and by hand searching. Of these, 530 records were irrelevant and duplicates. Among the remaining articles, 59 full-text articles were assessed for eligibility, of which 54 articles were excluded for the following reasons: inappropriate study type (n = 32), unusual diagnostic method (n = 12), and lack of a clear methodology or results (n = 10). Finally, 5 studies were included in this systematic review and meta-analysis (Fig. 1).
Characteristics of the Included Studies
In total, the 5 articles included in this study examined the effect of adding a statin to the standard treatment on the rate of H. pylori eradication. This effect was evaluated among 722 participants who were divided into two groups: the intervention group (standard regimen plus statin) versus control group (standard regimen). All 5 studies were randomized controlled trials. To detect the presence of H. pylori, endoscopic-based tests (rapid urease test with or without histology) were used in 4 studies and stool antigen test (SAT) in one study. As adjuvant therapy, simvastatin was used in 3 studies and atorvastatin in 2 studies. The proof of H. pylori eradication was assessed in 4 studies by the SAT and in 1 study using the urea breath test (UBT; Table 1).
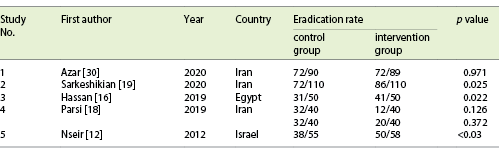
The addition of a statin to the standard treatment regimen significantly increased the rate of H. pylori in 3 studies. In 1 study, the increase in the eradication rate was not significant and in another study it paradoxically reduced the eradication rate (Table 2).
Meta-Analysis Results
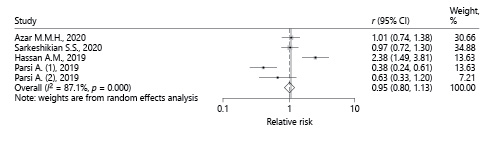
According to the I 2 index, which was equal to 88.2%, there is a considerable heterogeneity in the results of the studies included in this meta-analysis. Galbraith diagrams were used to determine studies whose results were not consistent with the other studies (Fig. 2), and we discovered that the results presented by Hassan et al. [16] and Nseir et al. [12] were not very consistent with the results presented in the other studies. Figure 3a was illustrated to determine the risk of bias according to the CONSORT checklist. As shown in Figure 3b, the asymmetry of the existing studies in the Egger funnel plot indicates potential publication bias. The results of the Egger test also confirmed this finding (p = 0.01).
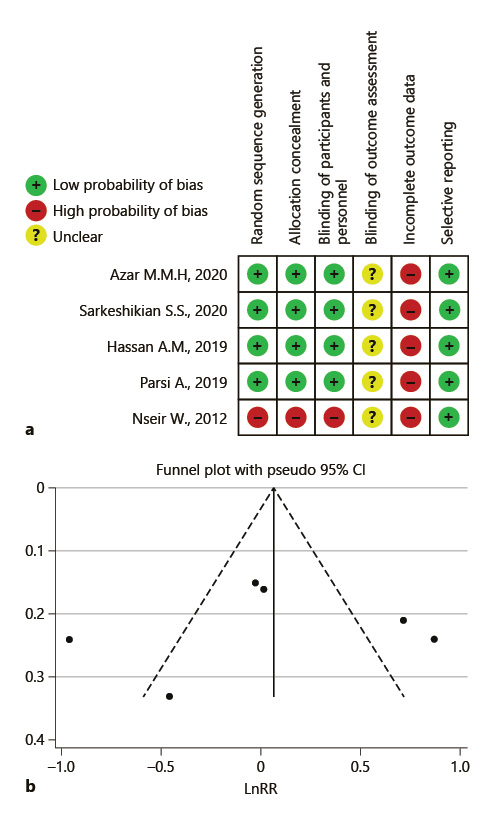
Fig. 3 Risk of bias. a Summarized authors’ judgment about risk of bias items for each included study. b Egger funnel plot of potential publication bias.
Due to the high heterogeneity among studies the random effects model was preferred. According to the random effects model, the value of the pooled RR was 1.03 (95% CI 0.64-1.68), which was not statistically significant (Fig. 4). Therefore, based on the meta-analysis of the findings of these 5 articles, the use of a statin had no significant effect on the H. pylori eradication rate. The χ2 test results show that heterogeneity is present in these studies (p < 0.001). However, due to the small number of studies included in this meta-analysis and the relatively small sample size of each of the analyzed studies, the ability of this test to detect heterogeneity is low.
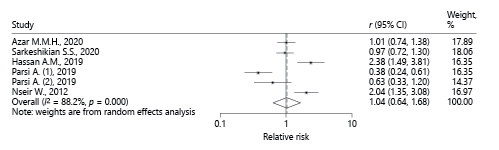
Fig. 4 Pooled RRof all studies describing the effect of statins on H. pylori eradication based on the random effects model.
Due to the difference in the administered statin type in the intervention groups of the studies (simvastatin and atorvastatin), and also variation in the assessment test (SAT and UBT), we also performed a subgroup analysis (Fig. 5, 6). Using the random effects model in simvastatin (I 2 = 92.7%, p = 0.000) and atorvastatin subgroup, the total RR (95% CI) was estimated to be 1.16 (0.92-1.48) and 0.99 (0.80-1.23), respectively. Combining the results of articles with SAT assessment test showed the pooled RR as 0.94 (95% CI 0.80-1.13). These results also determine that there is no significant change in H. pylori eradication rate after adding statins to the standard treatment regimen.
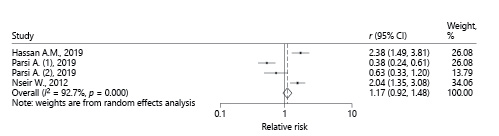
Fig. 5 Pooled RRof studies that administered simvastatin to the intervention group, describing the effect of statins on H. pylori eradication based on the random effects model.
Discussion
Although important progress has been documented in the management and treatment of H. pylori infection during recent years, achieving higher eradication rates and battling increased H. pylori resistance to medication remain major public health problems worldwide [17]. Unfortunately, the previously recommended first-line option which consisted of a triple therapy regimen (PPI + clarithromycin + amoxicillin) has failed to provide optimal results [14]. Several papers have evaluated the potential benefit of adding a statin to the standard therapy, but their findings were controversial [16, 18, 19]. On one hand, one study reported that statins, as adjuvants to the standard triple therapy, significantly improved H. pylori eradication rates [16]. On the other hand, another research documented a negative effect of statins on the H. pylori eradication rate [18]. In this meta-analysis of previously published studies in Iran, Egypt, and Israel, we evaluated the effect of statin addition to the recommended standard therapy on H. pylori eradication. Our results demonstrate that the addition of a statin to the current treatment regimen did not lead to a significant increase of the H. pylori eradication rate.
Supposedly, the potential benefit of statins in H. pylori eradication could derive from their anti-inflammatory and anti-microbial properties. For example, Jackson et al. [20] have evaluated the association between inflammation and H. pylori infection by measuring C-reactive protein (CRP) levels. Thus, in a population-based cross-sectional study of 2,633 randomly selected subjects, they assessed H. pylori presence based on serology and concluded after adjustment for age, gender, height, socioeconomic status, and smoker status that H. pylori infection is associated with an increased risk of having elevated serum CRP (>3 mg/L) levels. Furthermore, concerning the other speculated property of statins, namely their antimicrobial attributes, several papers have suggested that these lipid-lowering agents might be beneficial in the management of infection and even sepsis [21-23].
There are many discrepancies in the findings of the previous studies. According to Cheung et al. [24], statins might be effective in the chemoprevention of gastric cancer in H. pylori-eradicated patients. According to the newest findings, the success of H. pylori eradication therapies has declined in recent years, with 2 reports coming from the USA and Europe concluding that eradication rates remain <85% [25, 26]. The development of antimicrobial resistance can be a reason for this decline in eradication rates [27].
However, statin therapy might also involve certain drawbacks, such as the risks of gastroduodenal ulcer and reflux esophagitis, which have been reported as complications of these lipid-lowering agents. El-Hajj et al. [28] has discussed the case of a patient who developed atorvastatin-induced severe gastric ulceration after 3 months of therapy. In contrast, based on the results of an age- and gender-matched study involving 120 subjects newly diagnosed with gastroduodenal ulcer and 146 subjects with reflux esophagitis compared with 240 and 292 controls, respectively, Fujii et al. [29] concluded that the employment of statins is not associated with gastroduodenal ulcer or reflux esophagitis.
Based on this systematic review, we can conclude that an optimization of the H. pylori eradication strategy using statins remains an ongoing challenge. The studies encountered in the pool of available literature and analyzed in this paper have generated controversial findings. Furthermore, the pooled estimates of effect size in our meta-analysis did not support the benefit of statin addition to the available therapy in terms of improving H. pylori eradication rates. However, a final conclusion regarding statin effectiveness on eradicating H. pylori cannot be reached only based on the data available today. Consequently, further studies with sufficient sample sizes are needed to gather stronger evidence as to illustrate the effect of statin addition to the current treatment scheme employed for H. pylori eradication.
Although the main advantage of the present study is that we employed a meta-analysis to calculate the pooled estimate regarding the effect of statin addition to current treatment regimens used for H. pylori eradication, our study also has several limitations. The number of available studies which evaluated the effects of statins on H. pylori-eradication rates and have been entered into our meta-analysis is limited. In addition, most of the studies were performed in one center and involved a relatively small number of patients.