Introduction
Necrotizing fasciitis (NF) is part of a group of necrotizing soft tissue infections. It is a serious disease with fulminant evolution that appears as a vast necrosis of the subcutaneous tissue and superficial fascia.
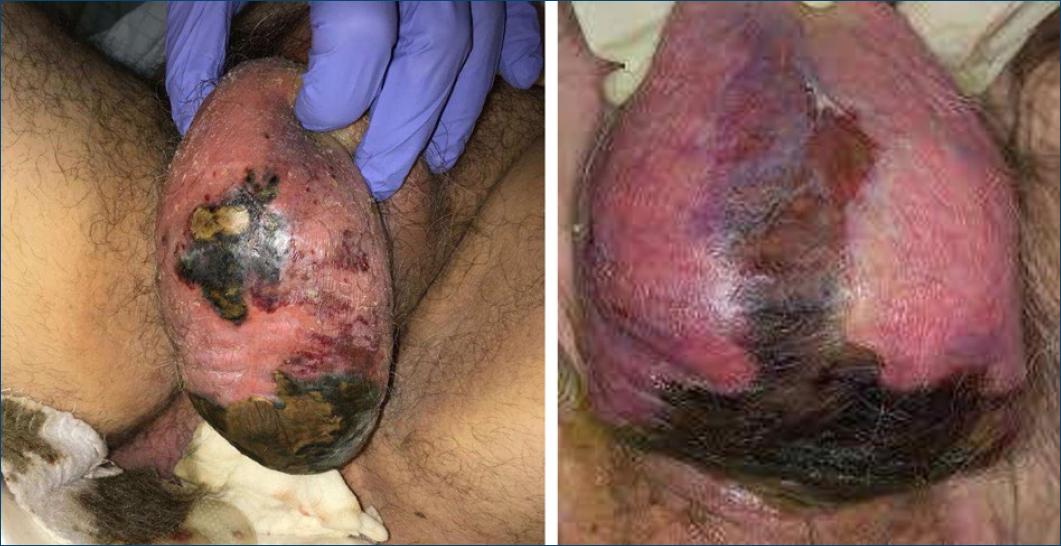
Necrotizing soft tissue infections can be classified according to the microorganism(s) at its origin. Type I is a polymicrobial infection in which the agents involved act synergistically for its development. Type II occurs due to group A Streptococcus (GAS), whether or not associated with methicillin-resistant Staphylococcus aureus (MRSA), and can progress to toxic shock syndrome (TSS). Type III appears in the context of infections by bacilli (e.g., Clostridium, Vibrium spp.) and type IV occurs due to a fungal infection (e.g., Zygomycetes, Candida spp.). It can also be categorized according to the depth of infection into necrotizing cellulitis (dermis and subcutaneous tissue), NF (fascial component) and necrotizing myositis (muscle), and according to its anatomical location, being named Fournier's gangrene (Fig. 1) when it happens in the perineal region and Ludwig's angina when it takes place in the submandibular space1-9.
At an early stage, it is difficult to differentiate NF from other skin and soft tissue infections and the basis for diagnosis is a high index of suspicion. Given its rapid evolution and the difficulty in establishing a timely diagnosis, there are high rates of morbidity and mortality.
The severity and complexity of this disease are undeniable. In an early stage of presentation, it may mimic less severe infections, such as erysipelas, and therefore this article aims to draw attention to this dermatological emergency, that is often underdiagnosed or even unrecognized by clinicians, in order to optimize the early institution of aggressive and appropriate surgical debridement, and thus improve the prognosis of patients with NF.
We performed a narrative review about type II FN, informing about its clinical manifestations, diagnostic and therapeutic approach, by analyzing recent and relevant scientific articles related to the topic.
Epidemiology
Necrotizing soft tissue infections are rare diseases, with NF being its most common form with an incidence of 0.3–15 cases per 100,000 inhabitants3. Despite advances in research practices, both on diagnosis and also on treatment, NF is associated with a mortality rate around 25–35% of cases and an amputation rate between 15 and 30%3,10. Mortality is even more pronounced in those who develop TSS or septic shock.
Necrotizing skin and soft tissue infections are more prevalent in males2,4,7. There are certain predisposing factors for the development of type II NF, such as non-penetrating trauma (contusion or muscle strain) and penetrating trauma (chickenpox lesions, insect bites, or intravenous drug use). It is usually diagnosed in citizens without comorbidities belonging to any age group2,5,7.
Microbiology and Pathophysiology
Type II NF is a monomicrobial infection that most commonly occurs due to GAS but may also be associated with MRSA infection.
The GAS has a diversity of virulence factors that potentiate tissue necrosis. These are the M protein, the most virulent of which are M-1 and M-3, streptolysins S and O, streptokinase, exotoxins A, B, and C, superantigens and hyaluronidase4,6.
Type II NF can occur with or without a port of entry. When the infection arises with a clearly identified portal of entry, infiltration of the organism or spores into the soft tissue is facilitated. Bacteria proliferate and release exotoxins that promote inflammation. These toxins induce the formation of platelet and leukocyte aggregates that at first occlude small capillaries, leading to edema and erythema, and subsequently larger venules and arterioles with consequent ischaemic tissue necrosis. When the disease occurs without an identified portal of entry, blood vessels cause the influx of leukocytes and myocyte progenitor cells, and the latter enhances the expression of vimentin on their cell surface and promote chemotaxis of GAS, which will proliferate in the tissues and produce exotoxins, following the cascade of events described previously1,3,5.
Clinical Presentation
In an early stage, the clinical presentation of type II NF may not be very evident, and patients may even be oligosymptomatic or asymptomatic, since the infection starts in the deeper layers of the skin, leaving its surface apparently normal, which makes its diagnosis at an early stage of presentation particularly challenging. This leads to delays in diagnosis, as well as in the implementation of adequate therapy, which reflects the significant morbidity and mortality associated with it.
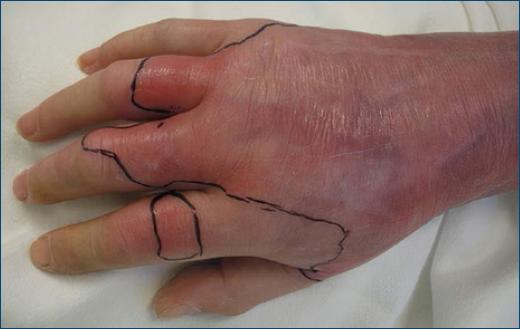
It is possible to characterize the signs and symptoms of type II NF as early or late and localized or systemic. Intense increasing pain disproportionate to the clinical presentation may be the first symptom to occur (in about 72% of cases)2,3,5,7,11. However, if the patient takes painkillers, it may be masked, further delaying the diagnostic process. Initially, patients present with edema beyond the margins of erythema (Fig. 2), allodynia, fever, and tachycardia. As the infection progresses, late signs and symptoms, which are also the most severe, begin to manifest. These include dark, violaceous skin discoloration (Fig. 3), hemorrhagic blisters (Fig. 3), ulceration, necrosis, discharge of brownish fluid compared in the literature to “dishwater,” especially after surgical debridement of the affected areas, hypoesthesia, sepsis, multi-organ failure, shock and death1-3,5,10. A meta-analysis published in 201812, aiming to report on the presence of fever, hemorrhagic blisters, and hypotension in the diagnostic acuity of necrotizing skin and soft tissue infections, concluded that these three clinical findings have a low sensitivity to identify them with fever, hemorrhagic blisters and hypotension being present on 46%, 25.2%, and 21%, respectively. Therefore, their absence at clinical presentation is not sufficient to exclude the disease.
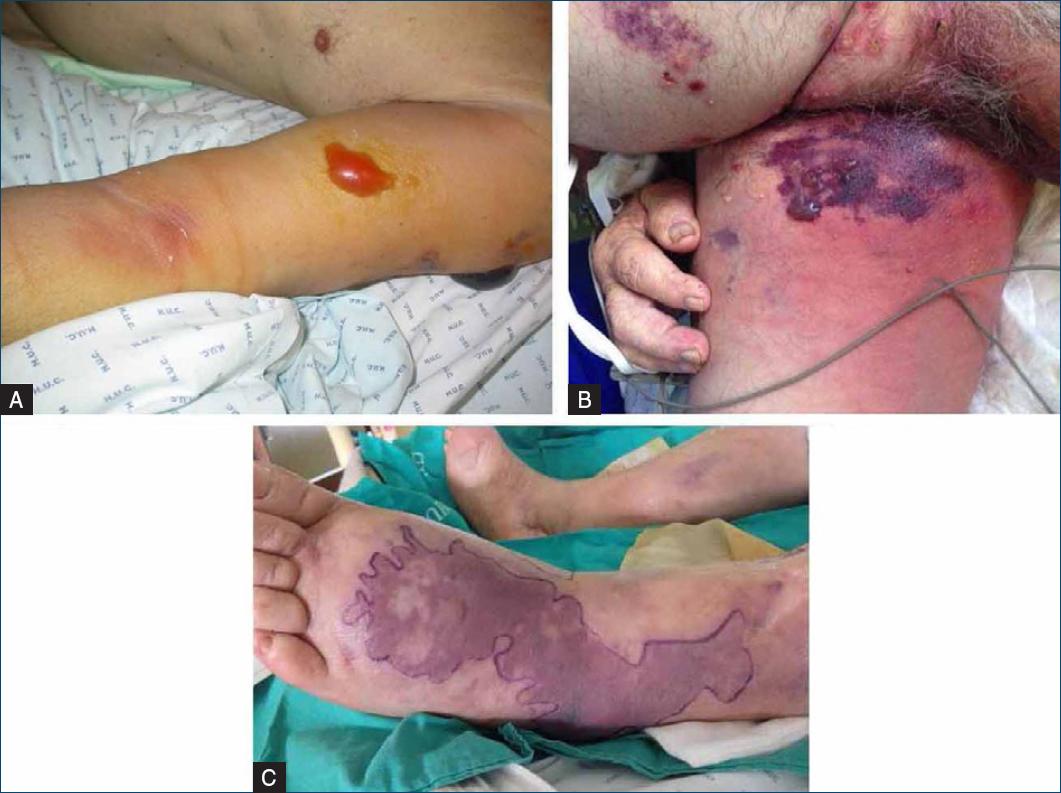
Figure 3 Late signs of type II NF: A: hemorrhagic blisters; B and C: violaceous discoloration in “geographical map” and formation of hemorrhagic blisters.
Evolution to TSS occurs in about 47% of cases13. To define this syndrome it is necessary to isolate the GAS, there must be hypotension (in the adult, a systolic pressure ≤90 mmHg and in the child, a systolic pressure less than or equal to the 5th percentile for that age) and there must be two or more features of multiorgan failure: renal involvement, coagulopathy (platelet count < 100,000 × 109 /L or presence or disseminated intravascular coagulation), liver involvement with elevated transaminases or bilirubin, acute respiratory distress syndrome, the presence of an erythematous macular rash, which may or may not be desquamative, and the presence of necrosis13-15. Patients with type II NF who develop TSS obviously have an even higher mortality rate (over 25% in the first 24 h and 34% in the first week). Therefore, the development of TSS is a predictor of poor prognosis7,16,17.
Diagnosis
Early diagnosis and treatment of NF are the cornerstones for optimizing the prognosis of the affected individual. The first one relies mainly on clinical presentation1,4,18, but in early stages of the disease, it may not be clear. Thus, the existence of complementary diagnostic tests that allow its initial recognition would be the key to overcome this difficulty.
Nevertheless, in case of a high suspicion of type II NF, no complementary diagnostic exams should delay surgical debridement, which is essential for the patient's survival1,3.
Finger sweep test
The simplest test, which can be performed at the patient's bedside and can confirm the presence of NF is the finger sweep test, which consists of a small surgical exploration of a suspected site of infection.
The finger sweep test is a simple technique done under local anesthetic. An incision of about 2 cm is made in the skin where the gloved index finger is inserted up to the deep fascia. Lack or minimal resistance of the fascia to dissection by the finger, absence of bleeding and the presence of brownish fluid with a foul smell, compared to “dishwater” or necrotic tissue at the time of incision indicate a positive finger sweep test and the presence of a necrotizing soft tissue infection1,18.
Triple diagnosis
Triple diagnosis should be performed in early stages of disease evolution when the degree of suspicion for NF is still low (assuming the patient's hemodynamic stability and time to perform it), or, in more advanced stages of the disease, if the delay from performing this technique does not compromise the beginning of the patient's surgical treatment. It includes an incisional biopsy, analysis of fresh tissue after frozen sections, and gram staining1,8.
The incisional biopsy is performed at the site of greatest suspicion of infection1,8. The tissue obtained is processed in order to obtain frozen sections that are stained by gram staining, which will identify the presence of bacteria in the tissue sample collected5,8.
The triple diagnosis is a fast-performing procedure and an excellent resource to diagnose NF. However, in practical terms, it may not be applicable, as not all hospital centers have access to a cryostat, which is indispensable for the freezing process. Also, the anatomopathologist is not always urgently available, and he is required for performing this procedure and interpretation of the results. This would delay not only the diagnosis, but also the surgical debridement1,2.
Surgical exploration, biopsy and cultures
When the suspicion of NF is high, surgical exploration remains the gold standard and should not be postponed in favor of further examinations.
Surgical debridement is performed immediately if macroscopic changes compatible with NF are seen or in the presence of a positive finger sweep test8. If macroscopic findings indicative of NF are not seen, intraoperative deep tissue biopsies are taken for diagnostic confirmation. A rapid frozen section analysis can be performed, depending on its availability.
In either case, biopsied tissue is sent for cultures and gram staining to identify the responsible infectious agent, which is crucial for future therapeutic adjustment8.
Imaging studies
When the clinical presentation of a patient raises doubts regarding the diagnosis, imaging studies can provide clues that allow the diagnostic hypotheses to be narrowed. However, if the suspicion of NF is high, surgical exploration is a priority and should not be postponed1,3,6,12.
Ultrasound is an advantageous complementary diagnostic tool because it is rapid and inexpensive, and it can be performed in the emergency department setting, at the patient's bedside and in individuals who are hemodynamically unstable. In addition, it provides information to help distinguish between simple and necrotizing soft tissue infections1, but in early stages of presentation it may not show obvious changes. Findings congruent with type II NF are the thickening of the subcutaneous tissue and the appearance of hypoechogenic areas corresponding to fluid accumulation along the deep fascia1,19,20. Increased echogenicity of fat may also be visible, however, this can also be seen in cellulitis21.
Plain radiography, despite being an easily performed and quickly accessible imaging modality, should not be used as a resource to exclude any type of NF, given its low sensitivity for the detection of necrotizing soft tissue infections12. Moreover, the main radiological finding is the presence of gas in the tissues, which does not occur in NF by GAS1-5,12.
Regarding computed tomography (CT), the indicative signs of type II NF are the presence of edema along the fascia as well as its thickening. Absence of enhancement of the fascia suggests necrosis of the affected tissue1,3,5,12. Magnetic resonance imaging (MRI) has been considered the best complementary imaging method for early recognition of NF, showing superior results when compared with CT1,3,8,12,18. However, the use of CT scan and MRI for diagnosing NF may delay surgical intervention, since they are not always available in a timely manner, take time to be performed and MRI is not done urgently, so they should not be used for this purpose when the index of suspicion is high1,3,8,12,22.
LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score
The laboratory risk indicator for necrotizing fasciitis (LRINEC) score (Table 1) was established in 2004 by Wong et al.18 to facilitate the distinction between NF and other skin and soft tissue infections using laboratory tests routinely performed during the initial evaluation of these diseases. This index stratifies patients in low, moderate, and high risk of developing NF, which allows a timely diagnosis of those most likely to develop the infection and, consequently, the institution of a therapy at the correct time. They concluded that this tool allows differentiating necrotizing from non-necrotizing infections, even in early stages of the disease, with a positive predictive value of 92% and a negative predictive value of 96%.
Table 1 LRINEC (laboratory risk indicator for necrotizing fasciitis) score
| Laboratory Data | Score |
|---|---|
| C-Reactive protein (mg/L) | |
| < 150 | 0 |
| ≥ 150 | 4 |
| Total white cell count (/mm3) | |
| < 15 | 0 |
| 15–25 | 1 |
| > 25 | 2 |
| Haemoglobin (g/dL) | |
| > 13,5 | 0 |
| 11–13.5 | 1 |
| < 11 | 2 |
| Sodium (mEq/L) | |
| ≥ 135 | 0 |
| < 135 | 2 |
| Creatinine (mg/dL) | |
| ≤ 1.6 | 0 |
| > 1.6 | 2 |
| Glucose (mg/dL) | |
| ≤ 180 | 0 |
| > 180 | 1 |
Total score: ≤5 points means a low risk of developing NF; 6–7 indicates a moderate risk of developing NF; ≥8 translates to a high risk of developing NF. Data adapted from Wong et al.18 and Medeiros Gomes et al.4.
Nevertheless, Wong et al.18 state that if there is a high clinical suspicion, surgical debridement should not be delayed despite the LRINEC score.
Later studies show dichotomous results regarding the application of this index.
Among the articles analyzed in this narrative review regarding the diagnostic acuity of the LRINEC score, three of them approved its use4,23 24. One of these concluded that, in symptomatic patients over a period of more than 8 h, it was possible to increase its sensitivity by decreasing the cut-off of 6, initially proposed by Wong et al.,18 to 423. Medeiros Gomes et al.4 assessed the discriminatory ability of the LRINEC index between complicated skin and soft tissue infections and necrotizing infections. They applied it to 282 patients, obtaining a sensitivity of 92.8%, a specificity of 31.3%, a positive predictive value of 13.4% and a negative predictive value of 99.5%.
Other studies have documented antagonistic results, concluding that, when used alone, the LRINEC score is not a good diagnostic tool to exclude NF12,25-27. Hansen et al.25 found no difference regarding the presence of septic shock and the risk of death within 30 days between patients with a LRINEC score of 6 or more and less. Neeki et al.27 investigated the reliability of the LRINEC index when applied in the emergency room, demonstrating a high false positive rate in patients with cellulitis and a high number of false negatives in individuals diagnosed with NF.
Given this asymmetry of results, the LRINEC score should be used with consideration in an adjunctive manner and not as a diagnostic tool. The patient's clinic and the doctor's judgment are more important than the total score obtained.
Treatment
In an initial approach, if the skin lesions are visible, they should be outlined with a skin marker7. Blood samples are taken for blood cultures and laboratory tests to be included in the LRINEC score. Fluid therapy and analgesia should be initiated1,2.
Antibiotic therapy
Early empirical antibiotic treatment is an important adjuvant to surgical debridement. It should be broad, covering gram positive, gram negative, and anaerobic agents, empirically assuming the presence of a polymicrobial form of NF, even though clinically, it may correspond to type II1,3,6,7.
The inclusion of an agent with action against MRSA is recommended, for example, linezolid, daptomycin, or vancomycin1,2,6. Vancomycin should not be given to individuals with renal impairment1. Additionally, a beta-lactamase inhibitor should be considered, such as piperacillin-tazobactam and ticarcillin associated with clavulanic acid, or a carbapenem (e.g., imipenem, meropenem, and ertapenem)3. Clindamycin or linezolid, due to their suppressive effects on exotoxin production, should also be included in the initial therapeutic regimen, particularly in patients showing evidence of TSS (Table 2)1-3,6.
Table 2 Early empiric broad spectrum antibiotic therapy
| Recommendation | Therapeutic regimen | Route of administration |
|---|---|---|
| 1st line | Vancomycin (25 ® 15 mg/kg Q12 h) + Piperacillin-tazobactam (4.5 g Q6 h) + Clindamycin (300–600 mg Q8 h) or Linezolid (600 mg Q12 h) + Piperacillin-tazobactam (4.5 g Q6 h) | Intravenous |
| If allergy to penicillin or cephalosporin | Vancomycin (25 ® 15 mg/kg Q12 h) + Aztreonam (2 g Q8 h) + Metronidazole (500 mg Q6 h) + Clindamycin (300–600 mg Q8 h) | Intravenous |
| If allergy to vancomycin | Daptomycin (4–6 mg/kg Q24 h) + Piperacillin-tazobactam (4.5 g Q6 h) + Clindamycin (300–600 mg Q8 h) | Intravenous |
Antibiotic therapy needs to be adjusted subsequently depending on the results of the cultures and the antibiotic sensitivity testing1-3,6,7,23.
Regarding type II NF, the recommended antibiotic therapy is the association of penicillin G and clindamycin (or linezolid in case of clindamycin resistance)3,7.
The duration of antibiotic therapy should be individualized and only discontinued when the patient reaches the dispensation of further surgical debridement, when clinically stable and apyretic for more than 48/72 h1,2.
Surgical approach
The surgical approach in patients with suspected necrotizing soft tissue infections is profoundly important. It should ideally be performed within the first 12 h of hospital admission, since, when delayed, it is associated with a higher mortality rate1.
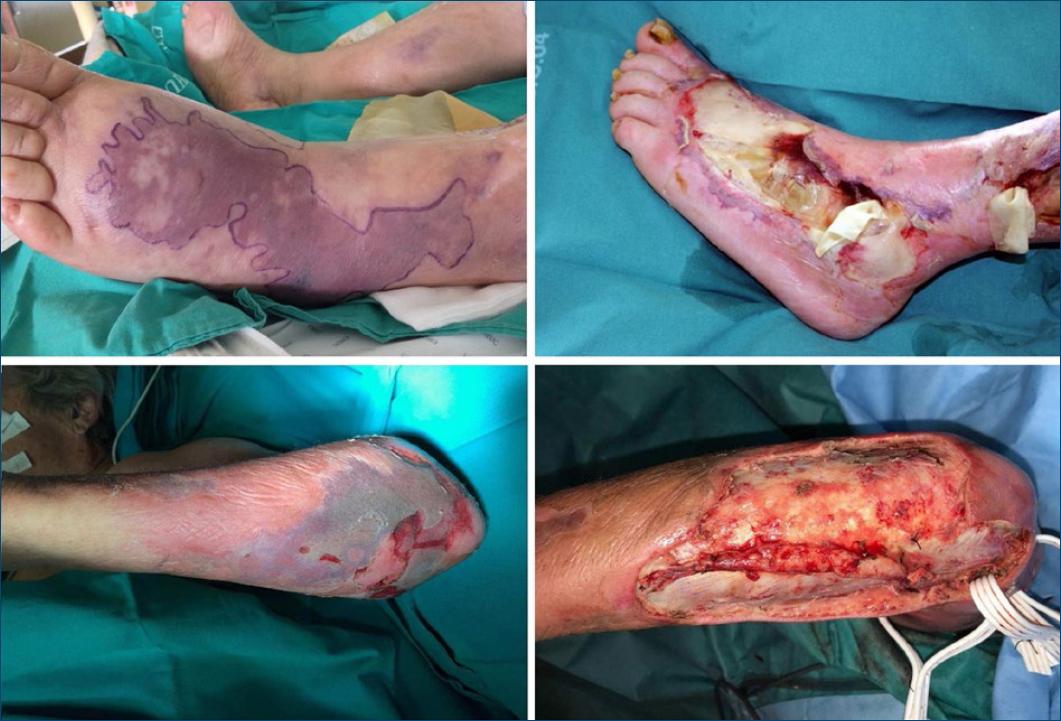
Early and complete surgical debridement plays an indispensable role for the prognosis of the affected patient1,2,5,6. This includes removal of infected fluids and debridement of all necrotic tissue until viable tissue is seen (Fig. 4)1,2,4.
As reiterated, surgical exploration is also crucial to obtain intraoperative biopsies. If the triple diagnosis has not been made prior to surgical exploration, these biopsies make the diagnosis of NF. Additionally, they are used to perform cultures and gram staining, in order to subsequently adjust the antibiotic therapy3,5.
After performing complete surgical debridement, surgical wounds should not be closed1,2,6,8.
Between 12 and 24 h after the first debridement, the patient should be submitted to a surgical re-exploration to verify the need for a new debridement1-3,5,6. The persistence of necrotic tissue implies its removal. These re-explorations and serial surgical debridements should only be discontinued when there is no more necrotic tissue1,3,5.
Negative pressure therapy should be considered to enhance the healing phase after completion of surgical debridement(s)1. It is a technique complementary to surgical closure that is based on the application of subatmospheric pressure to the surgical wound, combined or not with the instillation of antimicrobial lavage solutions. This procedure promotes faster wound healing and, consequently, early closure, which can be performed with autologous skin grafts (thin or full thickness)1. Pressure can be continuous or intermittent, the latter being recommended, as it induces the formation of granulation tissue to a greater degree compared to continuous pressure28.
Early initiation of nutritional support is recommended, taking into account the considerable protein loss and catabolic state these patients are in2-4.
Intravenous immunoglobulin
Recently, new therapeutic proposals have emerged for necrotizing skin and soft tissue infections. One of them is the use of intravenous immunoglobulin as an adjuvant to the treatment of type II NF. This hypothesis is based on its capacity to neutralize toxins produced by GAS2,3,5,6,16,29.
However, its therapeutic efficacy lacks scientific evidence, given the divergent results documented by different authors. Some have shown a decrease in mortality when using intravenous immunoglobulin as adjuvant treatment in patients with SGA infection with TSS1,2,6,16,29-31. On the contrary, other authors concluded that it had no therapeutic advantages1,5,32,33.
Thus, intravenous immunoglobulin, as an adjunctive treatment to surgical debridement and antibiotic therapy, may show some therapeutic benefit regarding the prognosis of the patient with type II NF and TSS, and is not advised for type I and III NF2. The recommended daily dose is 0.5–1 g/kg for 5 days2.
Hyperbaric oxygen therapy
Hyperbaric oxygen therapy was also presented as an adjuvant option for the treatment of necrotizing infections of the skin and soft tissues. Its use may only be considered in cases of type I NF and is not recommended for the remaining types1,2.
It is important to stress that the use of these new therapeutic modalities should not compromise early surgical debridement, as well as the initiation of antibiotic therapy, which are essential for the survival of patients with necrotizing skin and soft tissue infections1,5.
Conclusion
Although NF is an infrequent disease, it is associated with high rates of morbidity and mortality and, therefore, it is extremely important that the physician knows how to recognize it and act correctly3,10.
Type II NF arises due to infection by GAS, associated or not with MRSA, and can evolve to TSS. It is usually diagnosed in individuals of any age group without associated comorbidities2,5,7.
Initially, the clinical features of NF may not be evident, and patients may even be asymptomatic. This translates into a difficulty in establishing a diagnosis at an early stage of the disease. Progression to TSS occurs in around 47% of cases and is associated with a worse prognosis7,13.
The diagnosis relies mainly on the patient's clinical condition1,4,18. However, given its ambiguity in early stages, there are complementary diagnostic methods that help establish an early diagnosis. These include triple diagnosis, the finger sweep test and surgical exploration with biopsies for subsequent culture and gram staining1,8. Imaging exams, such as CT and MRI, may provide clues that help establish the diagnosis of NF, but when suspicion is high, they should not defer surgical exploration1,3,6,12,22.
Empirical antibiotic therapy and, especially, early and complete surgical debridement are the mainstays of NF treatment. Antibiotic therapy should be adjusted according to the agent(s) isolated, and for type II NF the combination of penicillin G and clindamycin is recommended1-3,6,7,23. Surgical treatment should begin within the first 12 h of hospital admission1. Around 12–24 h after the initial debridement, the patient should be submitted to a new surgical exploration and this cycle of re-explorations may only be discontinued when the absence of necrotic tissue is confirmed1-3,5,6.
To improve the prognosis of the patient with type II NF, it is fundamental that the doctor has a high index of suspicion in order to make the diagnosis and institute the correct therapy in a timely manner.
What does this study add?
This study compiles the most recent information regarding type II NF regarding epidemiology, microbiology, pathophysiology, clinical presentation, diagnosis, and treatment in order to allow an early diagnosis, as well as the institution of an adequate therapy, which are crucial for patient survival, by any physician facing this type of infection. Thus, by facilitating the early recognition of type II NF, it is possible to reduce the associated morbidity and mortality.