Introduction
Chronic spontaneous urticaria (CSU) is a common skin condition characterized by the development of itchy hives, angioedema, or both, with no recognizable triggers, lasting for a minimum of 6 weeks. It affects approximately 1% of the population worldwide1,2 with women being affected almost twice as frequently as males and a peak age at first symptoms between 20 and 40 years old3.
Although spontaneous remission is expected after an average period of 2-5 years4, extended periods of up to 10 years have been reported5. Moreover, CSU has a marked negative burden both on the patient and society due to the unpredictability of attacks, sleep deprivation, reduced performance at work or school, and limitation of social life and sexual dysfunction1. Thus, early, effective, and safe treatment is essential for this disease.
There are no curative treatments for CSU and current therapies aimed at symptomatic control are insufficient for many patients3. The first-line standard-dose or up-dosed 2nd-generation H1-antihistamine (H1-AH) is effective in less than half of CSU patients6, whereas the only other approved drug for CSU – the anti-immunoglobulin (Ig) E monoclonal antibody (mAb) omalizumab (OMA) – has a complete response rate under 70% according to real-world data7. Other medications such as cyclosporine, hydroxychloroquine, dapsone, and methotrexate are used off-label with variable efficacy8. In this context, the development of novel therapeutic options for CSU with increased efficacy is highly needed.
Encouraged by our growing understanding of the pathophysiology of CSU, several new treatment options are currently being studied in pre-clinical and clinical settings. These comprise biologicals, such as dupilumab (anti-IL-4Rα), secukinumab (anti-IL-17), tezepelumab (anti-TSLP), ligelizumab (anti-IgE), lirentelumab (anti-Siglet 8), and barzovolimab (anti-cKIT), and other drugs classified as “small molecules” in which Bruton tyrosine kinase (BTK) inhibitors (such as remibrutinib) and a Mas-related G protein-coupled receptor X2 (MRGPRX2) antagonist are included.
The aim of this work is to review the current and future therapeutic options for CSU that is targeting recognized pathophysiological mechanisms of the disease.
Basic pathogenesis of CSU
Urticaria occurs due to the activation and degranulation of mast cells and basophils, with consequent release of histamine, proteases, cytokines, platelet-activating factor (PAF), and other arachidonic acid metabolites (prostaglandin D2, leukotrienes C4, D4, and E4)3,9. These substances promote vasodilatation and increased capillary permeability, responsible for the hives and angioedema, as well as sensory nerve stimulation, which contributes to swelling, redness, and pruritus3.
Regarding CSU, autoimmunity and/or autoallergy are the main pathophysiological mechanisms involved in mast cell degranulation, but direct activation of mast-cell receptors as well as other inflammatory pathways together with the activation of the coagulation and complement cascades may also be involved in the development of lesions and symptoms of urticaria.
Autoimmunity and/or autoallergy
Two different subtypes of CSU are currently considered – type IIb autoimmunity and type I autoallergy9.
In autoimmune type IIb CSU, mast cells are activated by IgG targeting the high-affinity receptor for IgE on the surface of mast cells (IgG anti-FcεRI) or IgE bound to of mast cells (IgG anti-IgE). Up to 50% of CSU patients have these autoantibodies10 but their presence is not enough to define autoimmune CSU. According to a task force position paper published in 2013 and later confirmed by the PURIST study, in addition to the presence of IgG autoantibodies by immunoassay, autoimmune CSU requires also a positive BAT and positive autologous serum skin test (ASST)11,12.
In autoallergic CSU (autoimmunity type I), patients have IgE that recognizes autoantigens, such as thyroperoxidase (TPO), eosinophil peroxidase (EPO), IL-24, double-stranded DNA, tissue factor, FcεRI, and thyroglobulin. For some of these, namely IgE anti-IL-24 and IgE anti-TPO, in vitro and even in vivo activation of mast cells and/or basophils has been demonstrated13,14.
The existence of clearly defined and separate auto-IgE and auto-IgG CSU subtypes is still controversial13. Recent data suggest that IgG autoantibodies and other autoantibodies (IgE, IgM, and IgA) are co-expressed in the same patient, but actual overlap rates are still unknown15,16.
The central role of mast cells
Skin mast cells are the primary effector cells in urticaria, regardless of the subtype. Located predominantly in the upper dermis, they are increased in both lesional and non-lesional skin of CSU patients17. Detailed knowledge about their activating/inhibitory receptors, signaling pathways, and mediators helps in the identification of new potential treatment targets13.
Mast cells express several surface activating receptors including FcεRI, Mas-related G protein-coupled receptor PX2 (MRGPRX2), complement receptors (C5aR), protease-activated receptor (PAR)1, PAR2, and cytokine receptors (IL-4Rα and IL-5R), among others13,18.
Following the interaction of those receptors with their ligands, intracellular signaling is required for mast cell degranulation. Spleen tyrosine kinase and BTK are involved in the signal transduction downstream from FcεRI19. Apart from IgE/FcεRI-dependent signaling, IgE-independent pathways have increasingly been studied20. Mas-related G protein-coupled receptor X2 (MRGPRX2), for example, is an unselective receptor binding to many different agonists including endogenous neuropeptides (substance P), innate antimicrobial peptides, eosinophil granule proteins, and numerous synthetic drugs, such as codeine, some NSAIDs, and fluorquinolones21. Blocking MRGPRX2 seems promising not only for CSU but also for atopic dermatitis (AD), allergic contact dermatitis, non-histaminergic itch, and small molecule compound-induced pseudoallergy20.
Besides the activating receptors, there are also a few inhibitory receptors on the surface of mast cells, such as sialic acid-binding Ig-like lectin 8 (Siglec 8), which can block mast cell activation upon interaction with their ligands13.
Inflammation
In CSU, apart from mast cells, eosinophils, neutrophils, lymphocytes, and basophils are found around blood vessels, attracted to the skin in response to chemotactic factors, such as eotaxins, MCP3, RANTES, IL-5, C3a, C5a, TNF, IL-17, and PAF, which are released mainly by mast cells and activated endothelial cells13.
Basophils have a particularly important role in the pathogenesis of CSU, given the fact that, such as mast cells, they also release histamine, leukotrienes, and cytokines through activation of FcεRI and C5aR13.
The participation of eosinophils is also noticeable, mainly because of their bidirectional interaction with mast cells. Eosinophil granule proteins can induce mast cell degranulation and mast cell mediators (IL-5, TNF, PAF, and eotaxin) can activate eosinophils22. In addition, eosinophils contribute to the activation of the coagulation cascade by expressing tissue factor and they release MRGPRX2 agonists22.
Serum basopenia and eosinopenia are seen in 10-15% of patients with CSU, probably due to cell migration into the skin, and have been shown to be associated with higher CSU activity, presence of autoantibodies, and poor response to treatment13,23,24.
Although TH1 cells and TH17 cells are present, TH2 cells are the predominant type of lymphocyte in CSU. They stimulate IgE production and mast cell, basophil and eosinophil activation, by releasing many cytokines, namely IFNγ, TNF, TGFβ, IL-1β, IL-3, IL-4, IL-5, IL-6, IL-13, IL-17, IL-23, IL-24, IL-31, and IL-3313.
Coagulation cascade
In response to several mediators, eosinophils and dermal endothelial cells express high amounts of tissue factor on their surface, which activate the extrinsic coagulation cascade and leads to the production of activated coagulation factors13.
Coagulation factors, histamine, bradykinin, PAF, and/or other mediators act on vascular endothelial cells, either directly or through receptors (PAR1), promoting the formation of gaps between endothelial cells therefore increasing vascular permeability and allowing extravasation of plasma that may contain autoanti-bodies to IgE or FcεRI, and/or autoantigens for specific IgE bound to mast cells in the skin13.
Some activated coagulation factors, such as thrombin and FXa, may also directly induce mast cell activation, by acting on specific mast cell receptors (PAR1 and PAR2, respectively)13.
In addition, activation of extrinsic coagulation and fibrinolysis promotes the formation of complement components (C5a and C5b and/or C3a and C3b) that further activate mast cells and basophils, since both these cells express complement receptors (C3aR and C5aR) on their surface13. However, this hypercoagulative state in CSU is regarded mostly as a local process accompanied by active fibrinolysis without increased risk for thrombotic events25. Nevertheless, they may be related to the elevation of serum D-dimers26, which along with other serum biomarkers may be increased in CSU patients, namely interleukin-627, interleukin-1728, and C-reactive protein (CRP)29, whose levels apparently correlate with CSU activity. Furthermore, the proposed association between increased CRP, D-dimer levels, IL-6, C3, C4, ASST positivity, and CSU activity may reflect the complexity of this disease29, suggesting that autoimmunity, inflammation, complement activation, and coagulation are all somehow connected and continuously contributing for the maintenance and/or exacerbation of urticaria13.
Current management guidelines for CSU
Current treatment options proposed within the international EAACI/GA²LEN/EuroGuiDerm/APAAACI recommendations for the management of CSU (Fig. 1) are mainly symptomatic with the overall goal to “treat the disease until it is gone,” aiming at complete symptom control (UAS7 = 0 and/or UCT = 16) and a normalization of quality of life8.
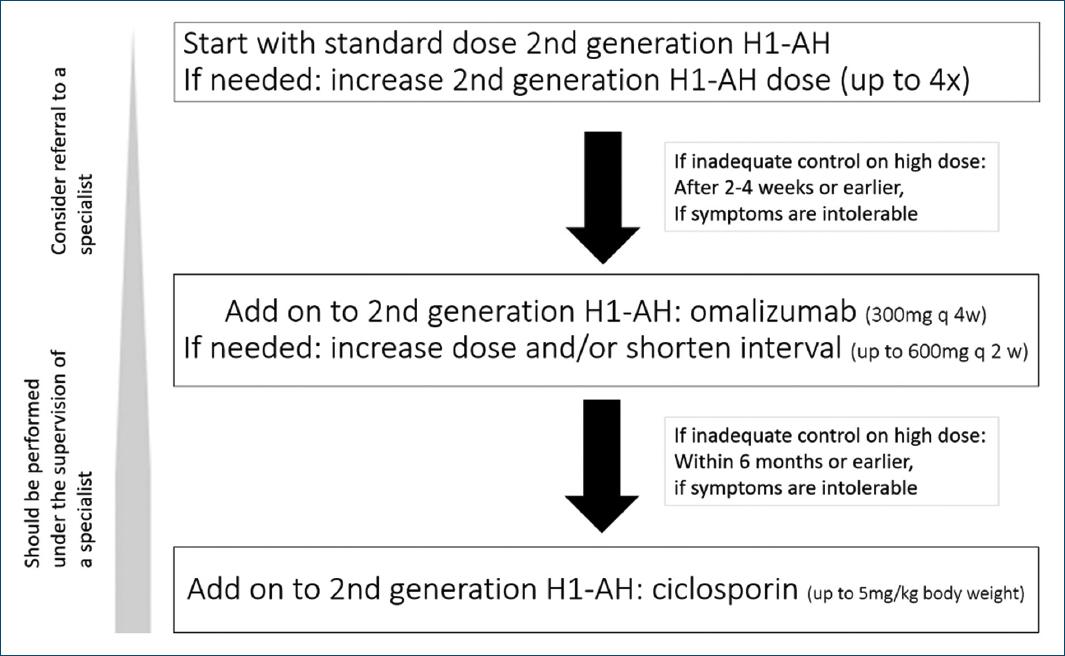
Figure 1 Current algorithm for CSU treatment according to the international EAACI/GA²LEN/EuroGuiDerm/APAAACI recommendations. Of note: in addition, a short course of glucocorticosteroids may be considered.
In addition to general measures, namely elimination of underlying causes and avoiding triggers such as stress or NSAIDs, recommended pharmacological therapy includes H1-antihistamines (H1-AH), OMA, and immunosuppressants, namely ciclosporin (CsA).
H1-antihistamines
H1-antihistamines have been recommended for the treatment of urticaria for more than 70 years7. They combine with and stabilize the inactive form of the H1 receptor, acting as inverse agonists and not as antagonists3,8.
First-generation H1-AH is strongly discouraged both in urticaria and other allergic disorders, because of their side effects (e.g., anticholinergic and sedative) and multiple drug interactions8,30. Non-sedating 2nd-generation H1-AH has a good safety profile, even at higher doses and after many years of continuous use, and is widely accepted as the first-line option for the management of CSU. They should be started in the standard dose and taken daily rather than on demand8. More than half of CSU patients cannot completely control their symptoms with standard doses6. According to several studies showing additional benefits of updosing 2nd-generation H1-AH in urticaria6,31, in patients with insufficient response, these drugs should have the dose increased up to fourfold before alternative treatments are considered8. Updosing is favored over mixing different 2nd-generation of H1-AH. Patients must be aware that 2nd-generation H1-AH updosing is off-label and higher than fourfold has not been tested therefore is not recommended8.
There are still no well-designed clinical trials comparing the effectiveness and safety of the different 2nd-generation H1-AH in urticaria, then so no suggestion can be made regarding which one to choose8.
In addition to updosing 2nd-generation H1-AH, the American guidelines for the management of urticaria also propose combining other therapies, such as H2-antihistamines, leukotriene receptor antagonists, or even a 1st-generation H1-AH at bedtime32. Those recommendations are mostly based on small case series and expert opinion and are not included within the main recommendation in the international EAACI/GA²LEN/EuroGuiDerm/APAAACI guideline8.
Omalizumab
OMA is a humanized anti-IgE mAb, which selectively binds and lowers free IgE, consequently decreasing FcεRI on basophils and mast cells due to the internalization and degradation of unattached FcεRI33,34. Reduced FcεRI expression seems to increase cells' resistance to IgE and IgG anti-FcεRI activation and therefore reduce histamine production and inflammation34.
Approved since 2014 for CSU in patients ≥ 12 years of age, OMA is still the only biological drug licensed for the treatment of CSU to date and is recommended as an add-on treatment after failure of antihistamine therapy at maximum doses13.
For the last decade, OMA has proven its efficacy and safety in both clinical trials and real-life studies35,36.
A recent meta-analysis of 67 real-world studies presented an average 25.6-point improvement in UAS-7 with OMA treatment (vs. a 14.9-22.1-point improvement reported in clinical trials). The same study reported a complete response rate of 72.2% and an additional partial response rate of 17.8%, as well as an average adverse event rate of 4.0% (vs. 2.9%-8.0% reported in clinical trials)37.
These data suggest that the efficacy of OMA in real-life practice may exceed what was previously reported in clinical trials, with a safety profile similar to or even better than the one observed during clinical trials37.
The benefits of OMA include not only the prevention of wheals and angioedema38 but also a remarkable increase in patients' quality of life39 and maintenance of efficacy in case of a relapse after suspension40.
In CSU, OMA should be started with subcutaneous injections of 300 mg every 4 weeks8. In patients who do not respond completely, OMA may be used in higher doses, shorter intervals, or both (up to 600 mg every 2 weeks)41-43, but patients must be warned that OMA updosing is off-label8. There are no definitive biomarkers to predict and explain why some patients have their symptoms controlled after the first injection whereas others take up to 6 months to respond44. Poor and/or slower response to OMA has been associated with type IIb autoimmunity45 and consequently, features of this specific CSU subtype – positive BAT/ASST44, low total IgE levels13,44, and basopenia/eosinopenia – have been postulated as possible predictors of a negative or slow response to OMA. Fast response is usually observed in patients with high serum IgE or when IgE increases after the first OMA injections46.
Most responders need long treatment periods until CSU remission, often more than 4-5 years, but it is hard to predict the right moment to discontinue OMA treatment and how to stop, either abruptly or progressively, increasing intervals between administrations until 6-8 symptom-free intervals. In case of a relapse after withdrawal, OMA has the same efficacy when re-initiated.
Ciclosporin
CsA is used off-label in CSU, in doses between 3 and 5 mg/kg/day, as a third-line treatment for patients without sufficient benefit from any dose of antihistamine and OMA in combination8.
CsA is an immunosuppressive drug which inhibits mast cell and basophil mediator release45. Its effectiveness in CSU has been demonstrated by several studies47-49 with response rates up to 73% in a recent meta-analysis48.
Predictors of favorable response to CSA have been explored and an association between type IIb phenotype and good/faster response to CSA has been proposed50,51. This means that patients with low IgE levels52 or positive BAT50 presumably have good responses to this drug, although, in a recent systematic review, no biomarker was consistent enough to be recommended49.
In addition in patients with this type IIb subtype of CSU, short courses of CSA have been shown to induce prolonged remissions in patients with CSU, without needing additional treatment53.
There are some concerns around the wider administration of this drug due to its poor safety profile, namely the risk of hypertension and cumulative renal impairment49.
Additional treatment options
In antihistamine refractory patients, the previously presented stepwise approach should be followed. However, guidelines and experts recognize that OMZ has limitations due to its high price and CSA may not be suitable because of adverse effects8. Therefore, in some cases, alternative treatments are needed.
Systemic corticosteroids (20 and 50 mg/d of prednisone equivalent) may be used in short courses (to a maximum of 10 days) and only for acute exacerbations of CSU, not in the long term8.
Other treatment options for CSU include dapsone, colchicine, sulfasalazine, hydroxychloroquine, methotrexate, tricyclic antidepressants, interferon, plasmapheresis, phototherapy, and intravenous Ig. These might be used in individual cases, but overall evidence to support their selection is weak13.
Therapies in development for CSU
Table 1 summarizes potential targets and agents under investigation for the treatment of CSU in adult patients.
Table 1 Potential targets and agents under investigation for the treatment of CSU in adult patients
| Target | Drug | Other names | Manufacturer | Trial phase | Trial identifier/designation | Status |
|---|---|---|---|---|---|---|
| IgE | Ligelizumab | QGE031 | Novartis | III | PEARL-1 PEARL-2 | Completed |
| UB-221 | United BioPharma | II | NCT05298215 | Recruiting | ||
| I | NCT04175704 | Not yet recruiting | ||||
| I | NCT04404023 | Not yet recruiting | ||||
| I | NCT03632291 | Completed | ||||
| IL-4/IL-13 | Dupilumab | REGN668/ SAR231893 | Sanofi | III | NCT04180488 (LIBERTY-CSU CUPID) | Recruiting |
| TSLP | Tezepelumab | Amgen | II | NCT04833855 (INCEPTION) | Completed | |
| IL-5 | Mepolizumab | GlaxoSmithKline | I | NCT03494881 | Recruiting | |
| IL-5Ra | Benralizumab | AstraZeneca | IV II | NCT03183024 NCT04612725 (ARROYO) | Completed | |
| Siglec 8 | Lirentelimab | AK002 | Allakos | IIa | NCT03436797 (CURSIG) | Completed |
| IIb | NCT05528861 (MAVERICK) | Recruiting | ||||
| KIT | Barzolvolimab | CDX-0159 | Celldex Therapeutics | Ib | NCT04538794 | Completed |
| II | NCT05368285 | Recruiting | ||||
| BTK | Fenebrutinib | GDC-0853 | Genentech | II | NCT03137069 | Completed |
| II | NCT03693625 | Terminated* | ||||
| Remibrutinib | LOU064 | Novartis | IIb | NCT03926611 | Completed | |
| III | NCT05513001 | Recruiting | ||||
| III | NCT05030311 (REMIX-1)/NCT05032157 (REMIX-2) | Active, not recruiting | ||||
| III | NCT05048342 (BISCUIT) | Active, not recruiting | ||||
| III | NCT05795153 | Recruiting | ||||
| Rilzabrutinib | PRN1008/ SAR444671 | Sanofi | II | NCT05107115 (RILECSU) | Active, not recruiting |
*Recruitment was stopped after an interim analysis of the parent GS39684 study.
Biologicals
LIGELIZUMAB
Ligelizumab (QGE031), a new generation humanized anti-IgE mAb, demonstrated a 40-fold to 50-fold greater affinity to IgE as compared with OMA53. Preliminary results of a phase IIb trial demonstrated rapid onset of action, dose-dependent efficacy, and superiority to OMA in refractory CSU patients54, but unfortunately, this was not confirmed by the following studies.
In PEARL-1 and PEARL-2 (NCT03580369 and NCT03580356), a phase III, replicate, multi-center, randomized, double-blind, parallel-group studies, which enrolled over 2000 patients aged 12 years or older with CSU refractory to H1-AH56, the primary endpoint was met (change from baseline in Urticaria Activity Score [UAS7] at week 12) but with no superior efficacy versus OMA. A good safety profile, consistent with previous studies, was reported55. Therefore, Novartis has stopped the development of ligelizumab for CSU.
UB-221
UB-221 is a recombinant humanized anti-IgE mAb distinct from OMA and ligelizumab since it neutralizes both soluble IgE and CD23-bound IgE56. A phase I study (NCT03632291) with a single UB-221 administration to patients with CSU has presented significant symptom relief along with a fast decrease in serum free-IgE level. Further phase I and phase II studies are being conducted to assess the efficacy, safety pharmacodynamics, and pharmacokinetics of this emerging intravenous drug56.
DUPILUMAB
Dupilumab is a recombinant human IgG4 mAb that inhibits IL-4 and IL-13 signaling by specifically binding to the IL-4Rα subunit of the IL-4/IL-13 receptor. It is currently approved for asthma, AD, chronic rhinosinusitis with nasal polyposis (CRSwNP), prurigo nodularis, and eosinophilic esophagitis (EoE)57.
Given the known predominance of type 2 inflammation in CSU, with IL-4 and IL-13 likely contributing to the inflammatory response through their effects on T-cell differentiation and IgE class switching, dupilumab has been postulated as a possible effective treatment in chronic urticaria58.
In fact, several case reports have shown efficacy in H1-AH and OMA-refractory CSU58, some with sustained benefits many months after suspension, suggesting a potential disease-modifying effect of dupilumab in CSU59.
The randomized, placebo-controlled, phase 3 clinical trial LIBERTY-CSU CUPID (NCT04180488) in H1-AH resistant CSU (study A and C: OMA naive; study B: OMA intolerant or incomplete responders)60 showed a clinically meaningful improvement in itch, hive severity, and urticaria activity at week 24, regardless of prior history of allergic rhinitis, asthma, AD, and normal and elevated IgE serum levels61. Nevertheless, contrary to positive results in OMA-naive patients (study A), dupilumab did not meet primary endpoints in patients who had failed OMA (study B), and the trial was stopped due to futility61.
Still, dupilumab seems to be a possible alternative treatment in biologic-naive H1-AH refractory patients and is currently being reviewed by FDA for that indication60.
SECUKINUMAB
Secukinumab is an anti-IL-17 mAb, widely used in moderate-to-severe plaque psoriasis, psoriatic arthritis, axial spondyloarthritis, and juvenile idiopathic arthritis.
As high serum and skin levels of IL-17 have been found in CSU, supposedly associated with high activity62, the role of IL-17 role in CSU pathogenesis has been suggested.
A recent study described the successful treatment of eight H1-AH and OMZ refractory CSU patients with secukinumab 150 mg once a week for 4 consecutive weeks followed by 150 mg every 2 weeks, although with a slow onset of action63. Future studies with larger numbers of patients are needed to confirm these results.
TEZEPELUMAB
Tezepelumab is a first-in-class human IgG2λ mAb against the action of TSLP. It is the only biological currently approved by FDA and EMA for severe asthma, and other indications, such as CRSwNP, EoE, and CSU, are under investigation64.
TSLP is an epithelial cell-derived cytokine that acts as an alarmin, promoting inflammation through the stimulation of dendritic cells, mast cells, and type 2 innate lymphoid cells62.
As increased TSLP expression has been demonstrated in the lesional skin of CSU patients and tezepelumab has proven to cause sustained decreases in circulating eosinophils and total serum IgE62, it has been postulated that this drug could be a useful alternative therapy for patients with H1-AH and OMA refractory CSU.
A 183-patient phase IIb trial to evaluate the efficacy and safety of tezepelumab in adults with CSU (INCEPTION; NCT04833855) has been recently completed65.
MEPOLIZUMAB AND OTHER ANTI-IL-5 MABS
Mepolizumab is a humanized mAb against IL-5, the key cytokine for the activation and survival of eosinophils, which is approved for the treatment of serious eosinophilic asthma, CRSwNP, hypereosinophilic syndrome, and eosinophilic granulomatosis with polyangiitis66. Considering the role of eosinophils in CSU, mepolizumab has been hypothesized as a valid option for the treatment of CSU67.
In 2018, Magerl et al. reported the case of a patient simultaneously affected by severe refractory eosinophilic asthma and CSU treated with mepolizumab, who showed good control of urticarial symptoms since the first administration68.
There is an ongoing interventional, single-arm, open-label, phase I clinical trial to evaluate the efficacy of mepolizumab in the treatment of CSU69.
Reslizumab, another anti-IL-5 biological approved only for severe eosinophilic asthma, has shown good efficacy in a single patient with severe asthma, CSU, and cold urticaria70. However, no further studies have been published.
Benralizumab, a murine mAb that binds to the isoleucine-61 of the domain 1 of human IL-5Rα, is licensed and used for severe eosinophilic asthma, but since it depletes eosinophils and basophils from affected skin, it may also improve symptoms of CSU67.
In phase IV, non-randomized, single-center trial (NCT03183024) with a total of 12 patients, CSU patients unresponsive to H1-AH treated with a single dose of subcutaneous placebo followed by 3 monthly subcutaneous benralizumab 30 mg injections had sustained mean changes in the UAS7 from baseline to week 2071.
Furthermore, a phase IIb multicenter, randomized, double-blind, parallel-group, placebo-controlled clinical trial (ARROYO; NCT04612725) to investigate the efficacy of benralizumab in H1-AH refractory CSU72.
LIRENTELIMAB
Siglecs (sialic acid Ig-like lectins) are I-type transmembrane proteins of the Ig superfamily found primarily on the surface of immune cells and are involved in inhibitory cell signaling. Siglec-8 is uniquely expressed on eosinophils, mast cells, and basophils and has been studied as a potential therapeutical target in CSU as well as in other types of chronic urticaria3.
Studies in mice have shown that monoclonal anti- bodies binding to Siglec-8, namely lirentelimab (AK002), inhibited MC degranulation, and cytokine production and induced eosinophil apoptosis73.
Results from a phase IIa trial (CURSIG; NCT03436797) reinforce that lirentelimab reduces disease activity in CSU patients, including those previously treated with anti-IgE therapy74. In this study, patients received 6 monthly intravenous infusions of lirentelimab and were followed for another 8 weeks. Both OMA-naive and OMA-refractory patients had a significant decrease in disease activity at week 22 (mean UAS7 change: −73% and −57%, respectively) without treatment-related serious adverse events74.
A phase IIb study to assess subcutaneous lirentelimab in patients with CSU is currently recruiting (MAVERICK, NCT05528861)75.
BARZOLVOLIMAB
Barzolvolimab (CDX-0159) is a humanized mAb developed to specifically inhibit the activation of KIT receptors by stem cell factor (SCF), which is essential for mast cell differentiation, proliferation, and survival76.
This drug was initially studied in chronic inducible urticaria, reaching 95% of complete responses in H1-AH resistant patients after a single 3 mg/kg intravenous administration. Tryptase suppression, skin mast cell ablation, and increased SCF were observed in accordance with the noticeable efficacy of the drug77.
A phase Ib trial (NCT04538794) with 45 patients to determine the safety of different doses of barzolvolimab in CSU showed rapid and lasting responses across multiple dosing groups (1.5 mg/kg, 3.0 mg/kg, and 4.5 mg/kg), with 56% of all patients experiencing complete responses at week 12, regardless of previous treatment with OMA77.
Multiple administrations of barzolvolimab demonstrated a favorable safety profile, consistent with single-dose studies, with mostly mild or moderate adverse events (hair color changes, COVID-19, headache, neutropenia, and urinary tract infections) with no need for drug withdrawal77.
The ongoing phase II clinical trial (NCT05368285) will provide further information on the therapeutic potential of barzolvolimab in CSU patients including those with prior biologic therapy.
Small molecules
MRGPRX2 ANTAGONIST
MRGPRX2 is a multiligand receptor, which promotes non-IgE driven mast cell degranulation, as well as neurogenic and eosinophilic inflammation78.
MRGPRX2-positive mast cells and some of their ligands (including substance P) are upregulated in the blood and/or skin of patients with pruritic skin diseases such as CSU or AD78, so it has been proposed as a promising therapeutical target for these conditions.
A preliminary study showed that EP262, a potent MRGPRX2 antagonist, can inhibit mast cell degranulation, both in vitro and in vivo79. This novel IgE-independent mechanism of action, with the potential for once-daily oral administration and a safety profile that is devoid of side effects, seems promising. A phase I first-in-human study will be initiated by Escient Pharmaceuticals to evaluate the safety, tolerability, and pharmacokinetics of the drug in healthy volunteers80.
BTK INHIBITORS
BTK, a non-receptor (cytoplasmic) tyrosine kinase, is involved in several immunological pathways, including signaling through Fce receptors but also through B-cell receptors, toll-like receptors, chemokine receptors, and CD4081.
BTK has a particularly important role in B-cell development and activation, which has motivated the development and approval of BTK inhibitors (namely ibrutinib) to treat B-cell malignancies81.
More recently, BTK has been found in many other non-B cells, such as mast cells, basophils, monocytes, and neutrophils, participating in several immunological pathways and the pathophysiologic mechanisms of inflammatory, autoimmune, and allergic diseases3,81.
In CSU, BTK is a key intracytoplasmic mediator of both type I and type IIb subtypes of CSU, not only because it is involved in mast cell degranulation but also because it mediates autoantibody production by B cells (Fig. 2). Interestingly, BTK has been proposed as particularly helpful in the more “treatment-resistant” type IIb CSU81.
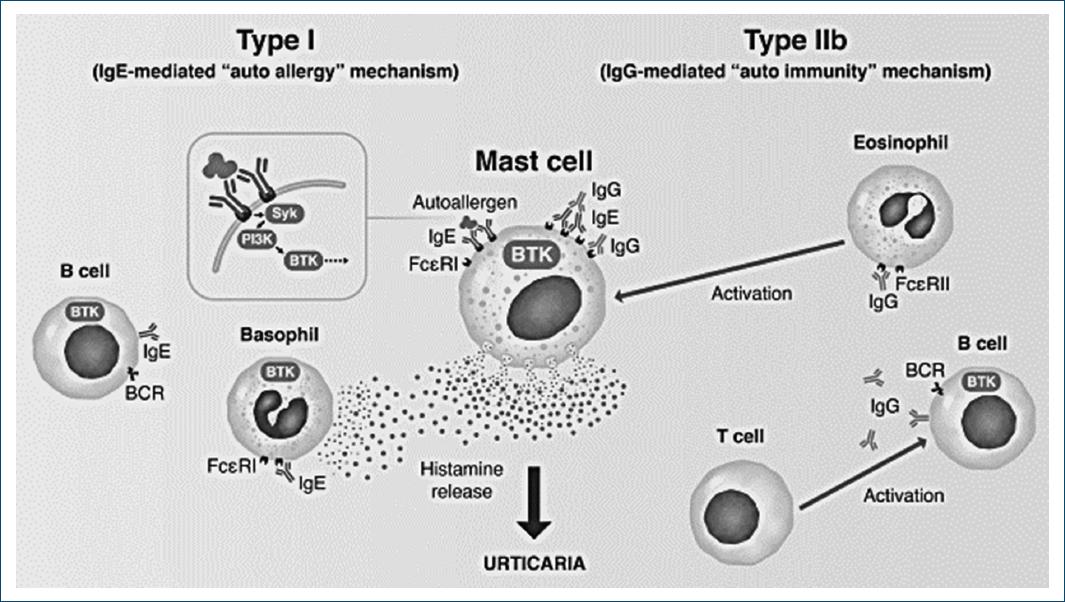
Figure 2 The role of BTK as an intracytoplasmic mediator of both type I and type IIb subtypes of CSU (adapted from: Mendes-Bastos P, Brasileiro A, Kolkhir P, et al. Bruton's tyrosine kinase inhibition – An emerging therapeutic strategy in immune-mediated dermatological conditions. Allergy. 2022;77:2355-2366).
FENEBRUTINIB
A recent double-blind, placebo-controlled, phase II study (NCT03137069) enrolled 93 participants to evaluate the efficacy, safety, and pharmacokinetics of the oral selective BTK inhibitor fenebrutinib for 8 weeks compared with placebo in H1-AH refractory CSU82.
Fenebrutinib 200 mg twice daily and 150 mg daily, but not at 50 mg daily, showed dose-dependent improvements in UAS7, especially in those patients with type IIb autoimmunity, but reversible grade 2 and 3 liver transaminase elevations occurred with these higher doses82.
REMIBRUTINIB
Remibrutinib, a highly selective, oral BTK inhibitor has been lately explored as a novel option for the treatment of CSU, obtaining promising results.
A randomized, double-blind, placebo-controlled phase IIb trial (NCT03926611), completed in 2022, evaluated the efficacy and safety of remibrutinib administered for 12 weeks in patients with CSU inadequately controlled with H1-AH, with or without prior exposition to OMA. Patients were randomized to one of six doses of remibrutinib – 10 mg i.d, 35 mg i.d, 100 mg i.d, 10 mg b.i.d, 25 mg b.i.d, or 100 mg b.i.d – or placebo, and at week 4 and week 12 all doses demonstrated superiority, with a rapid onset of action and independent of previous treatment with anti-IgE mAb and patients' baseline IgE83.
The median time to complete urticaria control (UAS7 = 0) was shortest with remibrutinib 25 mg b.i.d, which may be at least partially explained by the short half-life of the drug83.
Moreover, remibrutinib showed a favorable safety profile, with mostly mild or moderate adverse events and no apparent dose-dependent pattern83. Several phase III trials with this drug are currently in progress.
RILZABRUTINIB
Rilzabrutinib (PRN1008/SAR444671) is another oral small molecule inhibiting BTK which is currently under investigation for several conditions, including H1-AH refractory CSU. A phase II trial assessing the safety and effectiveness of rilzabrutinib in three different doses compared with placebo is ongoing and due to be concluded in 202484.
Conclusion
New therapies for CSU are urgently needed, given the high percentage of patients who respond poorly or not at all to currently available treatments.
This review summarizes the current treatment protocol for treating patients with CSU, as well as present novel drugs under investigation in clinical trials for this condition. Several promising therapeutic targets have been identified and individualized tailored therapies, based on the endotype and phenotype of each CSU patient, should be the future.
Several drugs are expected to be available in the next few years as add-on or alternative therapies for symptomatic control in patients who are resistant to H1-AH and OMA. A bigger ambition is the development of preventive or curative treatments, which may arise with the expanding comprehension of CSU pathogenesis.














