Introduction
Necrolytic migratory erythema (NME) is a rare skin disease typically associated with the glucagonoma syndrome, although it can be associated with other non-tumoral diseases such as nutritional deficiencies, chronic pancreatitis, or inflammatory bowel disease1. Glucagonomas are exceptionally rare tumors of the pancreatic alpha-cells, and their syndromic presentation includes NME, glossitis, cheilitis, venous thrombosis, diabetes mellitus, anemia, anorexia, and neuropsychiatric disorders2. NME treatment is closely related to oncologic treatment, with most cases showing improvement after successful tumor surgery, somatostatin analog therapy, or chemotherapy, in parallel with decreasing levels of glucagon or neuroendocrine markers1,3,4.
Clinical case
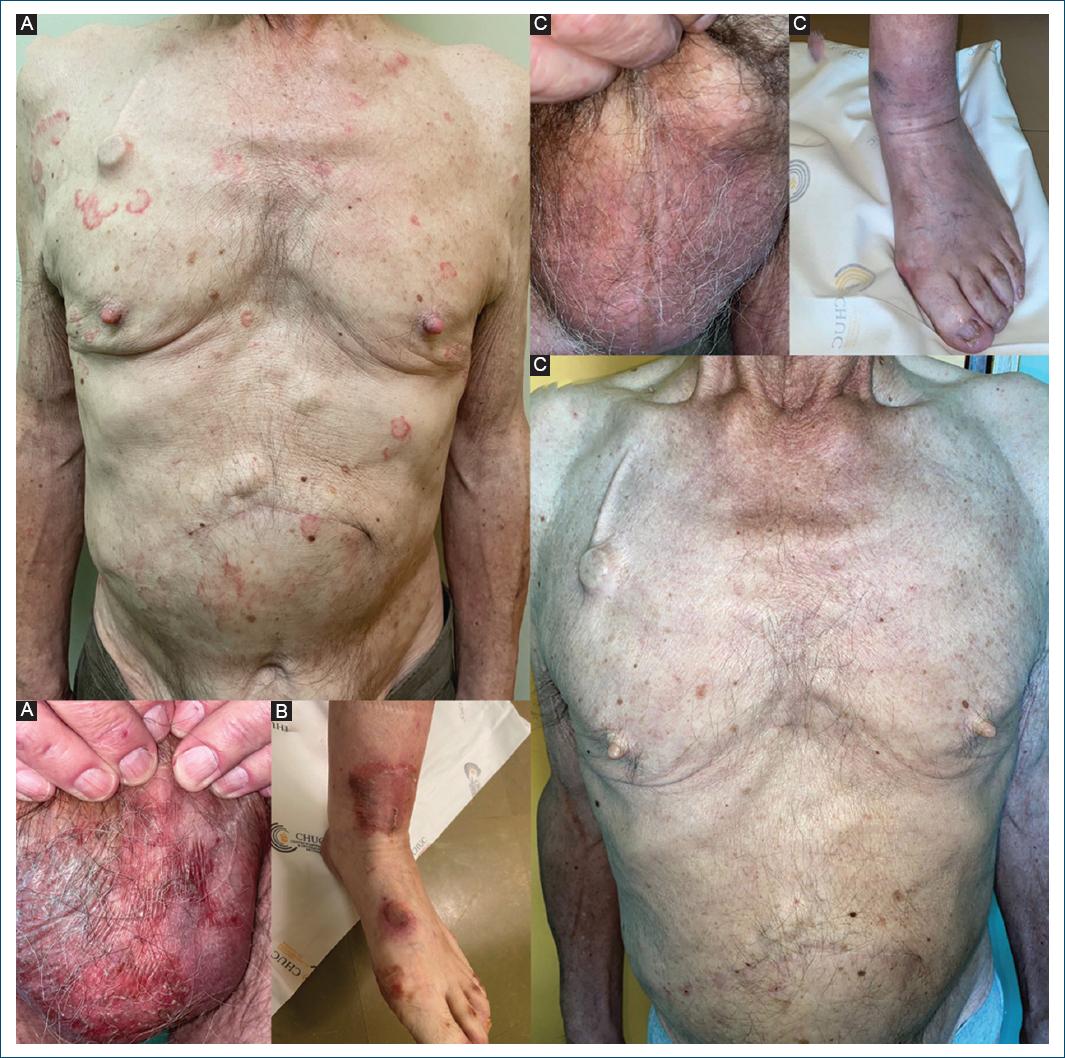
A 71-year-old man, with no other relevant medical history, was diagnosed with a stage II pancreatic neuroendocrine tumor. A few months after surgery, a positron emission tomography-computed tomography (CT) scan showed hepatic metastasis and suggested a local relapse of the primary tumor, later confirmed by hepatic magnetic resonance and thoraco-abdominopelvic CT scan. The patient was then subjected to thermal ablation of liver metastases and proposed for systemic therapy with the somatostatin analog drug lanreotide. One month later, the patient was referred to the dermatology department for a 2-year worsening dermatosis. He presented with polycyclic erythematous and scaly lesions, predominantly on the torso and upper limbs, also involving the genitals, with erythematous and vaguely erosive scrotal plaques (Fig. 1A). There was no mucosal involvement, and the patient showed an adequate nutritional status. After a non-diagnostic skin biopsy, there was worsening of the dermatosis and a subsequent histological evaluation showed confluent parakeratosis, keratinocyte vacuolization with neutrophil exocytosis, and a dermal lymphomononuclear perivascular infiltrate with scattered neutrophils, confirming the diagnosis of NME. Zinc levels were normal (0.7 mg/L, normal levels between 0.66 and 1.5 mg/L) and serum neuroendocrine markers were high (neuron-specific enolase 26 ng/mL, normal levels < 15 ng/mL; chromogranin A 350.7 ng/mL, normal levels < 85 ng/mL). Glucagon levels were also significantly elevated (1295 pg/mL, normal levels < 210 pg/mL). At the time of dermatology referral, the patient also showed progression of the disease on a CT scan. For that reason, by the time, NME diagnosis was confirmed, he began chemotherapy with capecitabine and temozolomide, maintaining lanreotide therapy. A month later, he maintained significant skin lesions, mainly affecting the lower limbs, with large erosive, crusted, and painful plaques, also involving the feet (Fig. 1B). Hepatitis C serology was negative. Zinc supplementation was initiated at a 220 mg twice daily dose for 2 months. At 3-month follow-up, there was a complete resolution of the dermatosis (Fig. 1C).
Discussion
NME pathogenesis is not yet fully understood. Hyperglucagonemia contributes to the dysfunction of the epidermis but most likely numerous aspects including hypoaminoacidemia or zinc and essential fatty acids deficiency are involved in the pathogenesis of NME. In our patient, after zinc oral supplementation, in the context of normal serum zinc levels, there was complete resolution of NME lesions that had persisted even after chemotherapy with concomitant somatostatin analog therapy. This has already been reported5, although rarely, and supports the role of zinc deficiency in the pathogenesis of NME.
Conclusion
In our patient, after zinc oral supplementation, in the context of normal serum zinc levels, there was complete resolution of NME lesions that had persisted even after chemotherapy with concomitant somatostatin analog therapy. This has already been reported, although rarely, and supports the role of zinc deficiency in the pathogenesis of NME.