Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies in women of reproductive age, affecting 5-20% of women worldwide1. This is a heterogeneous disease with a significant impact on a woman’s quality of life, often associated with metabolic, cardiovascular, and obstetric complications2. Although the diagnosis and early treatment are crucial for the quality of life and well-being of women with PCOS, most of them describe a delay in diagnosis, with multiple medical visits and inadequate health information until the definitive diagnosis3-5. In recent years, there has been an evolution in the classification criteria for this syndrome6. The first diagnostic criteria were proposed in 1990 by the National Institutes of Health (NIH)7, followed by the Rotterdam criteria proposed by the European Society of Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM)8,9. In 2006, diagnostic criteria were proposed by the Androgen Excess Society (AES)10,11.
PCOS can present with signs and symptoms associated with hyperandrogenism, menstrual irregularities, or polycystic ovaries on ultrasound12. Cutaneous manifestations such as hirsutism, acne, and androgenetic alopecia (AGA) are among the earliest manifestations of this syndrome, providing a clue for its diagnosis13. Therefore, as part of a multidisciplinary approach, dermatologists play an essential role in the early diagnosis and treatment of women with PCOS.
This review aims to evaluate the current knowledge on PCOS, highlighting the diagnostic criteria, dermatological manifestations, and therapeutic guidelines for managing this syndrome, considering that most of these women require long-term treatment and follow-up.
Epidemiology
PCOS is recognized as one of the most common endocrine disorders in women. Its prevalence depends, in part, on the diagnostic criteria used to define this condition, considering that each criterion includes a variable number of PCOS phenotypes.
In a 2018 systematic review and meta-analysis, Skiba et al. analyzed the prevalence of PCOS according to the NIH, EHSRE/ASRM, and AES classifications, and the overall prevalence of PCOS was 7%, 12%, and 10%, respectively14. A 2022 systematic review and meta-analysis described prevalence rates of 6%, 20%, and 15%, according to the NIH, EHSRE/ASRM, and AES criteria, respectively1. The differences reported by the two studies are partly due to the geographical areas included. In the most recent review, only studies in Europe or America were included, where similar prevalence rates were reported between the two continents1. Regarding PCOS in adolescence, prevalences of 3%, 11%, and 8% have been reported, according to the NIH, ESHRE/ASRM, and AES criteria, respectively15.
Pathophysiology
PCOS is a multifactorial disease with endocrinological, genetic, and environmental factors playing a role in its pathophysiology.
Regarding hormonal factors, this syndrome is characterized by hypothalamic-pituitary axis dysfunction and insulin resistance. Women with PCOS show an increased frequency of gonadotropin-releasing hormone pulses, resulting in preferential secretion of luteinizing hormone (LH). The relative increase in LH compared to follicle-stimulating hormone stimulates the production of androgens in the ovaries16,17. In addition, there is a decrease in insulin sensitivity, with consequent hyperinsulinemia18-20. In the ovaries, insulin stimulates the production of androgens by theca cells21,22 and, in the liver, inhibits the production of sex hormone-binding globulin (SHBG), increasing the bioavailability of androgens23. Therefore, the presence of these two factors contributes to the characteristic hyperandrogenism of this syndrome (Fig. 1).
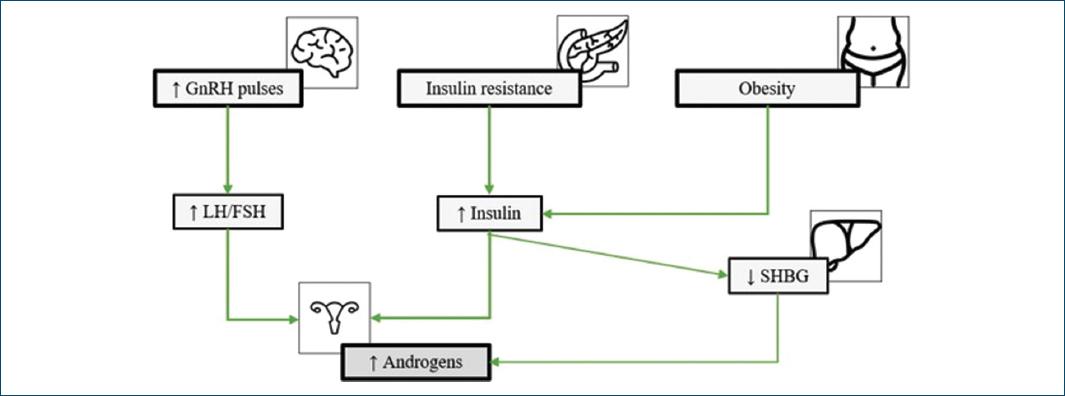
Figure 1 Pathophysiology of polycystic ovary syndrome. FSH: follicle-stimulating hormone; GnRH: gonadotropin-releasing hormone; LH: luteinizing hormone; SHBG: sex hormone binding globulin.
Obesity seems to contribute to the pathophysiological mechanism of PCOS due to its relationship with insulin resistance and consequent stimulation of androgen production, which contributes to the worsening of clinical manifestations of this syndrome24 (Fig. 1).
The interaction between genetic and environmental factors also plays a role in the pathophysiology of PCOS. Several studies among family members of women with PCOS have demonstrated the hereditary component associated with this syndrome, with an association of 70% among monozygotic twins25 and 20-40% among first-degree relatives26,27. However, PCOS is currently considered a polygenic disease, resulting from the interaction of multiple genetic and environmental factors. Several genes have been described in association with the action of gonadotropins and insulin, energy regulation, steroidogenesis, and folliculogenesis28,29.
Diagnosis
Diagnostic criteria
PCOS is a widely recognized condition, first described in 193530. However, due to its complexity, several diagnostic criteria have been proposed over the past three decades (Table 1). In 1990, the first diagnostic criteria defined by the NIH included the presence of chronic oligo-anovulation and hyperandrogenism7. In 2003, at a consensus held in Rotterdam, ESHRE and ASRM proposed the addition of a new criterion: the presence of polycystic ovaries on ultrasound. According to this classification, the diagnosis of PCOS requires the presence of two of the following criteria: clinical or biochemical hyperandrogenism, chronic ovulatory dysfunction, and polycystic ovaries on ultrasound8,9. In 2006, the EAS suggested a new definition of PCOS that included the presence of hyperandrogenism associated with ovarian dysfunction, such as the presence of oligo-anovulation or polycystic ovaries on ultrasound10,11. Due to controversies between the different diagnostic criteria, in 2012, the NIH consensus proposed maintaining the Rotterdam criteria (ESHRE/ASRM), with the identification of four phenotypes: phenotype A with the presence of all three criteria; phenotype B with the presence of hyperandrogenism and ovulatory dysfunction; phenotype C with the presence of hyperandrogenism and polycystic ovaries; and phenotype D with ovulatory dysfunction and polycystic ovaries31,32.
Table 1 Diagnostic criteria for polycystic ovary syndrome
| Classification | NIH 1990 | ESHRE/ASRM 2003 | AES 2006 | NIH ESHRE/ARSM 2012 |
|---|---|---|---|---|
| Criteria | ||||
| Hyperandrogenism (HA) | ✓ | ✓ | ✓ | ✓ |
| Chronic anovulation (CA) | ✓ | ✓ | ✓ | ✓ |
| Polycystic ovaries (PCO) | - | ✓ | ✓ | |
| Number of criteria | 2/2 | 2/3 | 2/2 | 2/3 |
| Phenotype | - | - | - | A: HA + CA + PCO B: HA + CA C: HA + PCO D: CA + PCO |
AES: Androgen Excess Society; ASRM: American Society for Reproductive Medicine; ESHRE: European Society of Human Reproduction and Embryology; NIH: National Institutes of Health.
Finally, the 2023 international recommendations for the management of PCOS endorse the Rotterdam criteria (ESHRE/ASRM), with a particularity: in the presence of menstrual irregularities and clinical and/or laboratory hyperandrogenism, ultrasound is unnecessary for the diagnosis12.
Diagnostic approach
If a teenager or woman presents with clinical manifestations of hyperandrogenism, such as hirsutism, acne or AGA, and menstrual irregularities, the diagnosis of PCOS can be assumed (Fig. 2). However, in the isolated presence of menstrual irregularities, it is advisable to request laboratory studies assessing plasma androgen levels, measuring free and total testosterone12. Androstenedione and dehydroepiandrosterone sulfate could be considered if total or free testosterone is not elevated, due to lower specificity of these hormone levels12,33,34. In women taking combined oral contraceptives (COC), this should be discontinued within 3 months due to its suppressive effect on gonadotropins and ovarian androgens12.
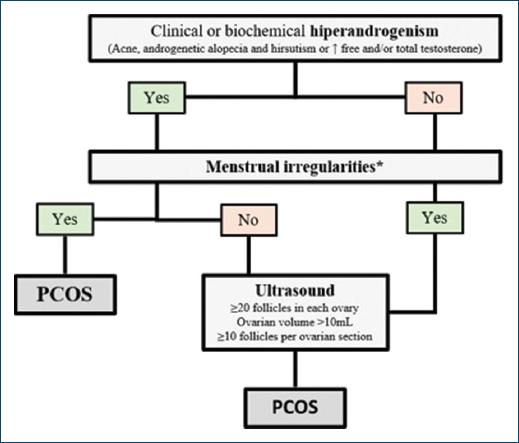
Figure 2 Diagnostic algorithm of polycystic ovary syndrome. PCOS: polycystic ovary syndrome. *Irregular menstrual cycles: normal in the 1st year after menarche; < 21 days or > 45 days if 1-3 years after menarche; < 21 days or > 35 days or < 8 cycles per year if 3 years after menarche; > 90 days for any cycle.
Ultrasound is only indicated for adult women with menstrual irregularities or hyperandrogenism. The diagnostic criteria for PCOS include the presence of ≥ 20 follicles in each ovary, ovarian volume > 10 mL, or ≥ 10 follicles per ovarian section12 (Fig. 2).
Regardless of classification criteria, diagnosing PCOS involves excluding other pathologies or etiologies that may mimic it. Therefore, the presence of hyperprolactinemia, thyroid pathology, or non-classical congenital adrenal hyperplasia should be ruled out. Therefore, all women should be screened with thyroid-stimulating hormone, prolactin, and 17-hydroxyprogesterone (Table 2). In the presence of other symptoms and according to clinical suspicion, other diagnoses should be considered, such as hypogonadotropic hypogonadism, Cushing’s syndrome, or androgen-producing tumours12,34-36 (Table 3).
Table 2 Laboratory screening tests for polycystic ovary syndrome diagnosis
| Blood tests for polycystic ovary syndrome |
|---|
| Free and/or total testosterone |
| Androstenedione* |
| Dehydroepiandrosterone sulfate* |
| Prolactin |
| Thyroid-stimulating hormone |
| 17-hydroxyprogesterone |
*Measuring androstenedione and dehydroepiandrosterone sulfate should be considered if testosterone or free testosterone is not elevated, according to the International Evidence-based Guideline12.
Table 3 Differential diagnosis for polycystic ovary syndrome
| Differential diagnosis | Laboratory studies |
|---|---|
| Thyroid disease | ↑/↓ TSH – hyperthyroidism/hypothyroidism |
| Hyperprolactinemia | ↑ Prolactin |
| Non-classical congenital adrenal hyperplasia | ↑ 17-hydroxyprogesterone |
| Cushing syndrome | ↑ 24-h urinary cortisol ↑ Late-night salivary cortisol Negative dexamethasone suppression test |
| Adrenal tumors | ↑ DHEA-S |
| Hypogonadotropic hypogonadism | ↓ LH e FSH |
DHEA-S: dehydroepiandrosterone sulfate; FSH: follicle-stimulating hormone; LH: luteinizing hormone; TSH: thyroid-stimulating hormone.
Dermatologic manifestations
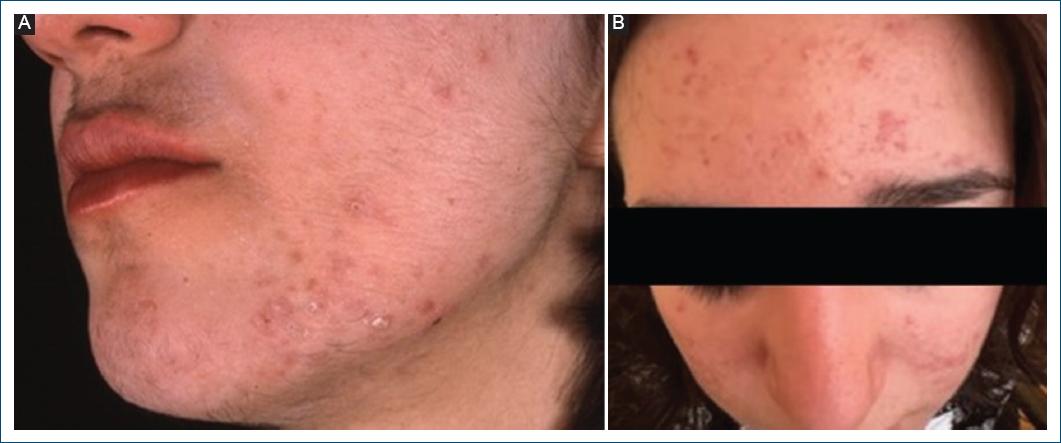
Dermatological manifestations of PCOS are a consequence of excess circulating androgens and may include hirsutism (Fig. 3), acne (Fig. 3), and37 (Fig. 4).
Hirsutism
Hirsutism is defined as excessive terminal hair growth in androgen-dependent areas in females and is one of the main manifestations associated with hyperandrogenism37,38.
The prevalence of hirsutism in women with PCOS varies between 47% and 90%, according to different studies13,39-42. In the hair follicle, there is an increase in the activity of the enzyme 5-α reductase, responsible for transforming testosterone into dihydrotestosterone (DHT), which is also stimulated by hyperandrogenism, insulin-like growth factor, and insulin43,44. Testosterone and DHT are involved in the hair cycle, transforming vellus hairs into terminal hairs in anatomical areas sensitive to androgens, such as the face, neck, or pubic area45,46.
The modified Ferriman-Gallwey scale is the most widely used method for classifying hirsutism. This scale scores the presence of terminal hair in nine female body areas, assigning a score between 0 and 4 for each47. A score ≥ 8 is indicative of hirsutism48,49 (Fig. 5).
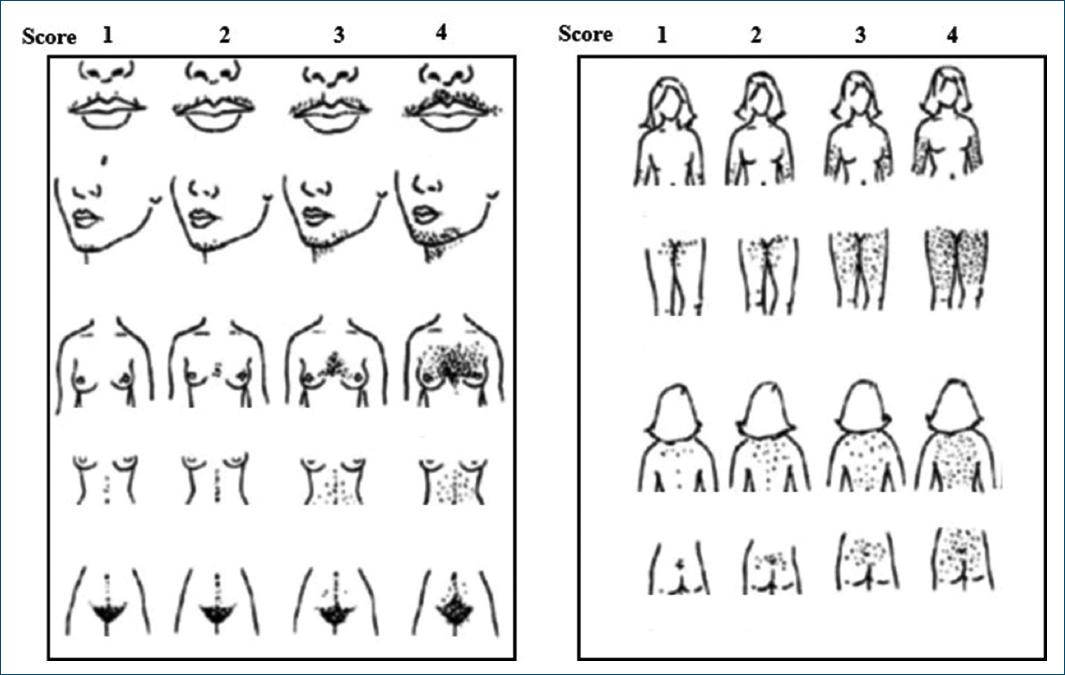
Figure 5 The modified Ferriman-Gallwey score for hirsutism. For each 9-body androgen-sensitive area, it is assigned a score of 0 (absence of terminal hair) to 4 (excess of terminal hair) (adapted from Hatch et al.38).
Acne
Acne is a multifactorial disease of the pilosebaceous unit and its pathophysiology includes follicular hyperkeratinization, inflammation, proliferation of Cutibacterium acnes, and increased sebum production. Genetic and extrinsic factors such as diet, medications, and environmental factors can also play a role in acne pathogenesis50. Androgens stimulate the growth of sebaceous glands, increase sebum production, and promote the shedding of follicular epithelial cells. These factors promote the formation of comedones, which, in association with colonization by C. acnes, trigger an inflammatory process, resulting in papules, pustules, nodules, or cysts43,51,52. Acne is also a marker of hyperandrogenism, with prevalences of 40-75% in women with PCOS13,39-41.
AGA
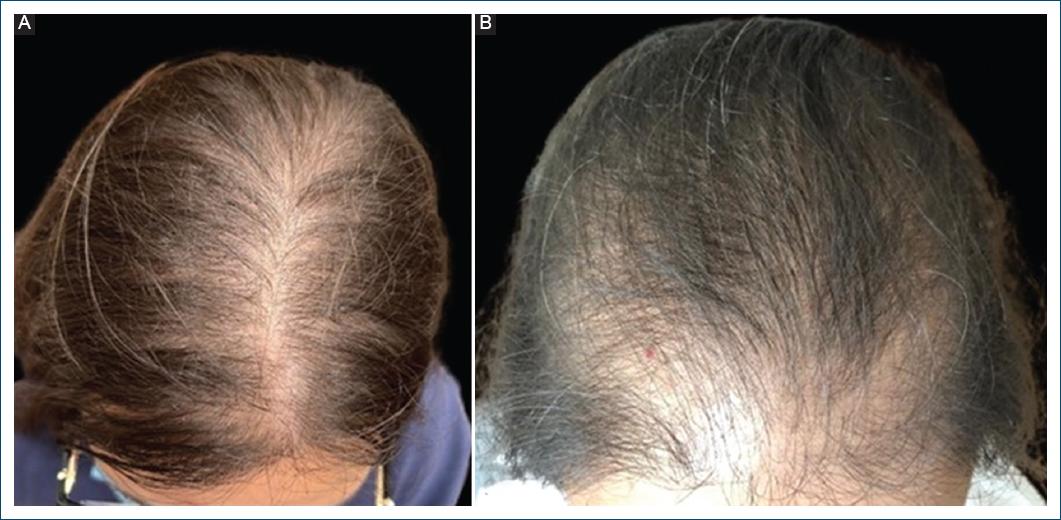
Female AGA is characterized by hair thinning in the central area of the scalp (Fig. 4A), due to the miniaturization of terminal hair into vellus hair and the shortening of the anagen phase, influenced by androgens46,53. This condition affects 20-43% of women with PCOS13,40,41.
The Ludwig scale is one of the methods used to classify this alopecia and ranges from I to III, according to severity54 (Fig. 6). Later on, the Olsen pattern was described, which is characterized by a marked hair thinning in the frontal hairline, known as the “Christmas tree” pattern55 (Fig. 4B).
Other dermatologic manifestations
Other PCOS cutaneous manifestations include seborrhea and acanthosis nigricans, the latter associated with insulin resistance56,57, with reported prevalences of 30-43% and 32-37%, respectively13,41.
Complications
PCOS is associated with metabolic, cardiovascular, gynecological, and psychological complications2,58 (Table 4). Due to insulin resistance, women with PCOS have an increased risk of glucose intolerance, type 2 diabetes mellitus, and metabolic syndrome, as well as an increased cardiovascular risk due to its association with a high prevalence of hypertension, dyslipidemia, and obesity59-61. Women with PCOS have a risk of up to 7 times higher of developing type 2 diabetes mellitus and up to 3 times higher of metabolic syndrome62,63.
Table 4 Non-dermatologic complications related to polycystic ovary syndrome
| Complications | Disorders |
|---|---|
| Metabolic | Glucose intolerance |
| Type 2 diabetes | |
| Metabolic syndrome | |
| Nonalcoholic fatty liver disease | |
| Cardiovascular | Obesity |
| Hypertension | |
| Dyslipidemia | |
| Gynecological | Infertility |
| Endometrial hyperplasia | |
| Endometrial carcinoma | |
| Psychological | Anxiety |
| Depression |
Due to chronic anovulation, women with PCOS may have difficulty conceiving, with PCOS accounting for 80% of cases of anovulatory infertility64. There is also an increased risk of endometrial hyperplasia and a 2-fold higher risk of endometrial carcinoma65-67.
Finally, the psychological consequences are associated with a negative impact on quality of life, with a higher prevalence of depression and anxiety in these women68,69. Women with PCOS have a four times higher risk of developing depression and a 6 times higher risk of developing anxiety70,71.
Therefore, the long-term health risks associated with PCOS highlight the relevance of early recognition of the syndrome, and dermatological manifestations may be among the earliest to be identified.
Treatment
Treatment of PCOS dermatological manifestations includes hormonal therapy aimed at reducing circulating androgen levels and inhibiting their action on target tissues (Table 5). In addition, there are various non-hormonal treatments for each manifestation that can be used as adjunctive or alternative therapies.
Table 5 Treatment options for polycystic ovary syndrome
| Drug | Dose |
|---|---|
| Oral contraceptives | Variable doses |
| Ethinylestradiol | |
| Cyproterone acetate | |
| Drospirenone | |
| Dienogest | |
| Desogestrel | |
| Gestodene | |
| Chlormadinone acetate | |
| Antiandrogens | |
| Spironolactone | 25-200 mg/day |
| Finasteride | 2.5-5 mg/day |
| Dutasteride* | 0.15-0.5 mg/day |
| Bicalutamide† | 25-50 mg/day |
| Flutamide† | 250 mg/day |
| Cyproterone acetate | Not recommended ≥ 10 mg |
| Metformin | 500-2000 mg/day |
Antiandrogens could be used to treat hirsutism if at least 6 months of COC and cosmetic therapy have failed to adequately improve symptoms. Irrespective of the drug used, at least 6-12 months of antiandrogen therapy is required for clinical improvement.
*For androgenetic alopecia.
†Increased risk of hepatotoxicity.
A healthy lifestyle is the treatment basis for this syndrome. There is evidence that diet, exercise, and weight loss are associated with an improvement in the clinical manifestations of the disease and a reduction in cardiovascular risk12,33,72.
Hormonal treatment
COCs are recommended in women of reproductive age for treating manifestations associated with hyperandrogenism but also to treat menstrual irregularities12. COC contains two active components: the estrogenic component and the progestative component73. The estrogen component stimulates the production of SHBG, decreasing the bioavailability of androgens, with ethinylestradiol being the most used. Regarding the progestative component, its androgenic properties vary according to the progestin, with cyproterone acetate being the progestine with the greatest anti-androgenic activity74,75. Other options may include COC containing a progestin with low affinity for the androgen receptor or a progestin with anti-androgen action, such as drospirenone, dienogest, desogestrel, gestodene, and chlormadinone acetate76-79. Guidelines do not suggest one formulation over another, due to the lack of clear evidence for differences in efficacy between preparations12,33,34.
COC should be prescribed considering their efficacy, availability, metabolic risk, and adverse effects. There are many formulations of ethinylestradiol and both high and low doses are equally effective. The main adverse effect is the increased risk of venous thrombotic events76. Therefore, COC with a high dose of ethinylestradiol and cyproterone acetate are not recommended as first-line treatment in PCOS12.
Antiandrogenic drugs should be considered when COC is contraindicated, poorly tolerated, or there is a suboptimal response after at least 6 months of treatment12. These drugs act by inhibiting androgen receptors, decreasing androgen production, or inhibiting the enzyme 5-α reductase. Its use requires effective contraception due to the risk of male fetus feminization if a pregnancy occurs80. The most widely used anti-androgen drug is spironolactone, with good tolerance and a low adverse effect profile. Other options include finasteride, cyproterone acetate, bicalutamide, and flutamide, the latter two being less commonly used due to their hepatotoxicity12,81,82. Dutasteride may be an option, especially indicated in AGA82. At least 6-12 months of antiandrogen therapy is required for clinical improvement83.
Metformin is an insulin-sensitizing drug that inhibits gluconeogenesis and lipogenesis and prevents weight gain by acting in the appetite regulatory pathways84. Metformin is recommended for metabolic indications in PCOS, while COC should be used for the management of hirsutism and irregular cycles12. A recent meta-analysis revealed that the combined treatment with metformin and COC improved biochemical hyperandrogenism, insulin levels, and insulin resistance more than COC alone84.
Non-hormonal treatment
HIRSUTISM
The management of hirsutism may include topical treatment or permanent hair removal techniques. Eflornithine hydrochloride is a topical drug that can be used as an adjuvant therapy due to its effect on inhibiting hair growth85,86. Permanent hair removal techniques include electrolysis and photodepilation with intense pulsed light or laser. Multiple sessions with regular intervals are required to achieve results87-89.
ACNE
Acne treatments are alternatives or adjuncts to hormonal treatment and may include topical treatments (retinoids, benzoyl peroxide, antibiotics, salicylic acid or azelaic acid, either as monotherapy or in combination, except for topical antibiotics, which should not be used as monotherapy)90 or oral therapy, namely antibiotics such as tetracyclines (doxycycline or minocycline) or isotretinoin, an oral retinoid that targets all four pathogenic factors of acne90.
AGA
AGA treatment includes topical or oral therapy. Topical minoxidil is the first-line treatment for women with AGA. Its therapeutic effect can be enhanced by oral drugs such as spironolactone, a drug with anti-androgenic potential, or finasteride, an inhibitor of the 5-α reductase enzyme91,92. Dutasteride is a potential alternative to finasteride because it is more potent at inhibiting 5 alfa reductase93. Other options include oral minoxidil or topical finasteride in combination with topical minoxidil. Other therapeutic modalities include platelet-rich plasma injection94, low-level laser therapy95, as well as hair transplantation if there is a poor response to pharmacological treatment93,96,97.
Conclusion
PCOS is one of the most common endocrine disorders in women of reproductive age and is associated with several long-term risks with a significant psychological impact on adolescents and women. It is a multifactorial condition that requires a multidisciplinary approach. Dermatological manifestations such as hirsutism, acne, or AGA play a key role in its diagnosis, and the dermatologist is crucial in its management and treatment. Hormonal treatment aims to minimize the effect of androgens on the skin and hair follicles. Several non-hormonal treatments can be used as adjunctive or alternative therapy.