Folate is naturally present in many foods, including vegetables, fruits, beef liver, nuts, beans and peas.
Folic acid is a synthetic form of vitamin B9 and it is chemically distinct from natural folates. It is metabolically inactive and needs to be metabolized to 5-methyl-THF, after which it behaves identically to natural dietary folate1. It can be incorporated into food during production process to enhance grain-based products such as bread, flours, pastas, rice, and cornmeal, and can also be present in certain dietary supplements.
The recommended daily amount of folate for adults is 400 micrograms2. In order to have higher bioavailability at the intestinal brush board, the synthetic form of folic acid only contains the monoglutamate conjugate3.
Adequate levels of folic acid are needed during pregnancy to help prevent neural tube defects such as anencephaly and spina bifida. Women who are planning a pregnancy are advised to take 400 μg of folic acid daily, starting at least 1 month before pregnancy4.
Hypersensitivity reactions to an essential food componente such as folic acid are rare. Only a few cases have been reported in the literature, with the first one described in 19495.
We present a 38-year-old woman with a history of an allergic reaction following the initiation of oral folic acid intake for pregestational supplementation.
In 2021, 30 minutes after the first intake of a 5mg folic acid tablet (Folicil 5mg, BIAL) as recommended by her Obstetrician, she experienced palmoplantar pruritus, generalized urticaria, periocular angioedema and conjunctival hyperemia. She promptly took an oral antihistamine with no clinical improvement and was taken to the Emergency department where she was treated with intravenous steroids and antihistamines, with complete symptom resolution after a few hours. Upon discharge she was advised to avoid folic acid supplements and was referred to the Allergy and Clinical Immunology Department for further investigation.
The patient’s personal and family history was unremarkable and denied any prior adverse drug reactions, food allergy (including food that are fortified with folic acid) or other allergic diseases. Following a thorough anamnesis and after providing informed consent, the patient underwent a diagnostic workup, consisting in skin prick test (SPT) and a basophil activation test (BAT).
Dilutions were prepared under laminar flow, grinding pills of folic acid (Folicil 5mg, BIAL) to a very fine poder and proceeding to sequential dilutions in sterile 0.9% saline solution. Suspensions were prepared at 5mg/mL (1:1) for SPT3,5,9 and at 0.05mg/L (1:100) and 0.03123mg/mL (1:160) to perform the BAT, considering the solubility of the folic acid6. Dilutions at 1:100 and 1:160 were submitted to sterilizing double filtration at the end of preparation with filter 0.22 μm.
The SPT with a 5 mg/ml suspension resulted positive with a wheal of 6 mm. Five control subjects underwent SPT with the same dilution and had a negative result. For that reason, no IDT were done. To perform the BAT, we used a 0.05 mg/ml (1:100) and a 0.03125 mg/ml (1:160) solutions and the analysis was made by flow cytometry, using the FLOW CAST® Basophil Activation Test (Flow cast, Buhlmann, City, Switzerland) according to the manufacturer’s instructions. Basophils were gated based on the constitutive expression of the chemokine receptor CCR3, using a monoclonal antibody labelled with phycoerythrin (CCR3-PE). The activation marker CD63 (gp53) expression on basophils was measured using an anti-CD63 labelled with fluorescein isothiocyanate (CD63-FITC), before and after cell stimulation.
Results were reported as percentage of basophils expressing CD63 and the ratio between stimulated and non-stimulated cells. Positivity was defined as >5% of basophils expressing CD63 and a stimulation index (SI) >2.
The patient’s BAT with folic acid was considered positive for both solutions: 0.05 mg/ml (1:100) (CD63=9.03%; SI=10.1) and 0.03125 mg/ml (1:160) (CD63=7.46%; SI=8.4) (Figure 1).
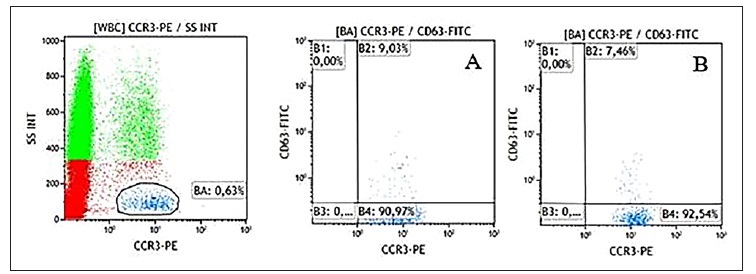
Figure 1 CD63 expression on basophils stimulated with folic acid in both solutions - A (1:100) and B (1:160)
The diagnostic workup favors a diagnosis of an IgE mediated allergy to folic acid. The patient was advised to abstain synthetic folic acid and keep a diet rich in natural folates to provide adequate nutrition. Since then, the patient did not have any other episode of urticaria/angioedema.
Despite the widespread use of folic acid supplements, there have been very few reported cases of allergy. Reactions to folic acid have been described after oral and intravenous administration with clinical manifestations ranging from recurrent urticaria to anaphylactic shock5.
In most cases where folic acid allergy is reported, patients tend to tolerate folic acid from natural sources. This suggests that the active folic acid ingredient in natural foods may have been rendered non-allergenic by food processing or that antigens are either absent or concealed in these products7.
Another hypothesis suggests that dietary folic acids, in their polyglutamate form, have lower bioavailability8.
Most of the reported cases in the literature were substantiated by positive SPT3,5,9,10,12. Although the low molecular weight of folic acid prevents it from being recognized as a full allergen when isolated, its ability to produce a positive immediate skin test suggests that is capable of rapidly combining with self-proteins or polypeptides in the skin to form a complete allergen10.
Currently, BAT is applied in research settings, and it can be a useful and safe complementary in vitro test, allowing to confirm the diagnosis without the risks of na oral provocation test, especially in subjects experiencing serious systemic reactions11. However, the diagnostic accuracy of BAT with folic acid requires further study.
Oral provocation tests with folic acid have been described12.
However, in this case, given the patient’s history consistent with an immediate reaction and positive results from the diagnostic workup, an oral provocation test was not performed.
In conclusion, folic acid allergy is a rare condition. We present a patient exhibiting an immediate reaction to folic acid, supported by positive results both from SPT and BAT, suggesting an IgE-mediated allergy. To our knowledge, this is the first reported case describing the application of a BAT in folic acid allergy.















