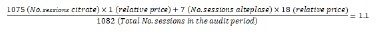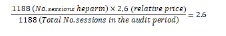INTRODUCTION
Tunneled catheters are a widely used vascular access in hemodialysis patients. These are frequently complicated by catheter-related thrombosis, which potentially compromises dialysis adequacy and may limit cateter survival.1,2 Catheter malfunction due to occlusion or thrombosis can have a significant impact on patient morbidity and dialysis resources. Moreover thrombolytic interventions add additional financial burden to the already escalating costs of chronic hemodialysis therapy. Strategies to prevent hemodialysis catheter dysfunction and infection include catheter locks.
The principle of the catheter lock is to instill an anticoagulant solution into the lumen of the branches of the hemodialysis catheter after each hemodialysis session, leaving them in place until the next session.3
The most widely used lock solutions for hemodialysis catheters are heparin and citrate. Heparin exerts its anticoagulant effect by binding to antithrombin and antagonizing anti-Xa and anti-IIa activity, as well as all activated coagulation factors, while citrate anticoagulante effect depends on its ability to chelate calcium, which is fundamental to the activation of the coagulation cascade, but also of platelet activity.
The use of heparin as routine locking solution is associated with several risks: systemic heparin administration occurs even when the catheter lock volume is limited to the volume of the catheter lumen or recommended fill volume; heparin-induced antibodies may occur; it interferes with specific lab studies, like prothrombin time. Current data predominantly favors citrate locks for the prevention of catheterrelated bloodstream infection, with less consistent results regarding thrombotic risk and preserving catheter patency.4-7
In November 2020, our hemodialysis unit converted to locking all central venous hemodialysis catheters with sodium citrate 4% instead of heparin 5000 U/mL. A retrospective analysis was conducted to evaluate whether replacing heparin with sodium citrate 4% would ensure cost-effective long-term catheter function and patency.
METHODS
Study design
This was a retrospective cohort study carried out in Davita Sacavém hemodialysis unit. Two treatment strategies were compared: a cateter locking solution composed of heparin and a catheter locking solution composed of sodium citrate 4%.
Our hemodialysis unit converted to locking all central venous hemodialysis catheters with sodium citrate 4% instead of heparin 5000 U/mL as unit policy change. All subjects received a sequence of treatments.
In the first period, the heparin period, all catheters were locked with concentrated heparin 5000 U/mL; in the second period, the citrate period, all catheters were locked with sodium citrate 4%.
Two 6-month audit periods were selected: six months prior and 6 months after the conversion from heparin to citrate locking. All patients in whom a central venous hemodialysis catheter was used during at least part of each period were included in the analysis, hence, the same population was used in the heparin and citrate period. All catheters were tunneled and double lumen. Daily monitoring of all patients was performed in accordance with standard practices for patients undergoing renal replacement therapy. The process for locking catheters was identical with the heparin solution and the sodium citrate 4%: at the end of each hemodialysis session, the lumens of the cateter were flushed with 10 mL of normal saline. Next, the locking solution was instilled slowly into each lumen as a locking agent in a volume corresponding to luminal capacity.
Exclusion criteria included patients with a contraindication to systemic anticoagulation, documented or suspected heparin-induced thrombocytopenia or known allergy to citrate.
Age, gender, dialysis vintage, comorbidities, catheter characteristics and patient outcomes were analyzed. The compared outcomes and their definition were as follows: incidence of catheter thrombosis measured by number of treatments with alteplase; number of cateter exchanges due to catheter dysfunction; dialysis adequacy was measured by the KT/V of all hemodialysis sessions; prevalence of hemodialysis sessions with catheter dysfunction defined as inability to achieve and maintain a blood flow of more than 350 mL/min despite changing the patient’s position, inverting the lines and flushing with saline solution; catheter-related bacteremia defined as fever or cold chills and at least one positive blood culture and no other obvious cause of infection; exit-site infections defined as the development of a purulent exudate or redness around the site not resulting from residual stitches.
Unintended reactions to the locking solution were recorded. Finally, the financial data was compared for each of the audit periods. The cost included the price of locking solutions and materials.
Ethical considerations
Procedures of the study were considered by the ethical committee of the hemodialysis clinic to be in accordance with the ethical standards on human experimentation and with the Helsinki Declaration.
Statistical analysis
Quantitative variables are presented as mean ± standard deviation (SD) when normally distributed or as median (interquartile range (IQR)) if non-normally distributed. Qualitative values are presented as number (percentage).
The Wilcoxon matched-pairs signed-rank test, a non-parametric test, was used to compare the rates in the parameters under review. A two-sided p-value of <0.05 was considered statistically significant.
RESULTS
Baseline characteristics of patients
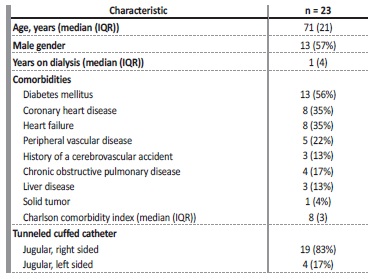
Out of 165 patients in our hemodialysis clinic during the year of study duration (May 1st 2020 - May 31st 2021), 23 (14%) were dialyzed using a central venous catheter during both periods of the study and included in this study. Only 4 patients were dialyzed using a central venous catheter during the full 12 months. The remaining patients were dialyzed through a central venous catheter during a period of time in both the heparin and citrate periods. There was a total of 1188 hemodialysis sessions in the heparin period (with a median catheter duration of 138 (112) days) and 1082 hemodialysis sessions in the citrate period (median catheter duration of 126 (131) days).
Table 1 includes the characteristics of the patients. Ten were female, 13 were male with a median age of 71 (21) years. Median dialysis vintage was 1 (4) years. The patients had multiple comorbidities, with a median Charlson comorbidity index of 8 (3). The most prevalente comorbidities were diabetes mellitus (13; 56%), coronary heart disease (8; 35%), heart failure (8; 35%) and peripheral vascular disease (5; 22%). Regarding catheter characteristics, 19 (83%) had a right jugular vein tunneled catheter, the remaining 4 (17%) had a left jugular vein tunneled catheter. These 23 patients were dialyzed using a central venous hemodialysis catheter during both the heparin and citrate lock period, so the patient characteristics are the same in both groups.
Catheter patency and dialysis adequacy
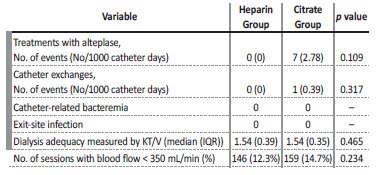
The rate of eparin thrombosis measured by number of treatments with alteplase was 0 (0 per 1000 catheter days) and 7 (2.78 per 1000 eparin days) during the eparina and citrate periods, respectively (NS, p=0.109). The number of eparin exchanges due to eparin dysfunction was 0 (0 per 1000 catheter days) during the eparina period and just 1 (0.39 per 1000 catheter days) during the citrate period (NS, p=0.317). The total of 7 alteplase treatments and 1 catheter eparina were performed in the same three patients
Dialysis adequacy was measured by the KT/V of all hemodialysis sessions. Median KT/V was 1.54 (0.39) in the eparina group and 1.54 (0.35) in the citrate group (NS, p=0.465). Regarding eparin dysfunction (inability to achieve and maintain a blood flow of more than 350 mL/min), it was recorded in 146 sessions (12.3%) in the eparina period and in 159 sessions (14.7%) in the citrate period (NS, p=0.234) (Table 2).
Infectious complications
There were no catheter related infections, namely catheter-related bacteremia or exit-site infections, in either group.
Adverse events and bleeding episodes
No serious adverse events that could be attributed to the locking solution were reported in both groups.
Cost of locking solutions
The relative cost of locking solutions per treatment, which included the price of locking solution and materials, was calculated at 1 for sodium citrate; 2.6:1 for heparin and 18:1 for alteplase (Table 3). When including the cost associated with alteplase therapy, the relative price of locking solutions per hemodialysis session was 1.1 in the citrate period and 2.6 in the heparin period (Figure 2). This reflects a 58% reduction in the costs associated with catheter-locking therapy in the citrate period (Figure 1), including the costs associated with thrombolytic therapy.
DISCUSSION
The prevalence of patients dialyzed through central venous catheters remains substantial. Portugal has a tunneled catheter prevalence of 19%,8 compared to the 14% prevalence observed in our clinic.
The use of a central catheter is associated with a multitude of potential complications. Infection, catheter thrombosis and associated malfunction are among the primary complications associated with central venous hemodialysis catheters.3 A variety of hemodialysis catheter locking solutions are used to prevent catheter thrombosis, the most commonly used are heparin and sodium citrate 4%. Current evidence favors citrate lock solutions in reducing infection complications and bleeding episodes, with less consistent results regarding risk of thrombosis or catheter removal due to poor flow.7 With this evidence in mind, KDOQI - Kidney Disease Outcomes Quality Initiative considers reasonable the choice to use either citrate or heparin as a CVC locking solution.1
When comparing catheter lock with heparin versus citrate, this study did not find any statistically significant differences regarding major catheter related outcomes: rate of catheter thrombosis, rate of catheter exchange, catheter dysfunction or dialysis adequacy. It is important to note that all alteplase treatments and catheter Exchange occurred in three patients during the citrate period. When patients transitioned from the heparin to the citrate group, the catheter dwelling time was longer, which can be associated with higher rate of thrombotic complications.
It was not possible to study infection related events such as catheter-related bacteremia or exit-site infection because of the absence of these events.
A strength of our study is that the same population was used in the heparin and citrate period, minimizing confounding covariates like patient or catheter characteristics. We acknowledge that the small number of patients enrolled and the retrospective nature are limitations of this study. Other limitations are the low number of events and the limited follow-up time in each group.
In our hemodialysis unit, the direct cost of citrate locking is considerably less than the cost of heparin locking. There was a 58% cost reduction associated with catheter-locking therapy between the heparina period and the citrate period. This cost reduction included the costs associated with alteplase use.
In addition to the financial advantages of switching to citrate locking, there are other potential benefits, namely the minimization of heparin related side effects or limitations. Citrate improves reliability of INR assays and can be used in cases of confirmed or suspected heparin induced thrombocytopenia.9 It is important to note that citrate can also cause systemic complications, namely cardiac arrhythmia; however, these are rare in the 4% concentration.
In conclusion, citrate locking is an effective and less expensive alternative to heparin locking of hemodialysis catheters, that does not appear to be inferior to heparin. Since the conclusion of this study, the continued and successful use of citrate as a routine locking solution in our hemodialysis unit has been quite positive.