Introduction
Over the past two decades, chronic kidney disease (CKD) has become a leading cause of global mortality and stands out as one of the few non-communicable diseases with a notable increase in related deaths.1 The rise in the prevalence of CKD can be attributed in part to the increasing influence of risk factors such as obesity and diabetes mellitus. This progressive disease now affects more than 800 million people, accounting for 10% of the world’s population. As of 2017, an estimated 843.6 million people worldwide were living with CKD.1,2 The impact of CKD is particularly severe in low- and middle-income countries, where resources to tackle its consequences are limited. Despite a decline in mortality rates for people with end-stage kidney disease (ESKD), studies from the Global Burden of Disease (GBD) indicate that CKD is a leading contributor to global mortality. It is imperative to identify, monitor, and treat CKD, while implementing widespread preventive and therapeutic measures. These efforts are critical to addressing the escalating health problem posed by CKD on a global scale.3
Diabetes, cardiovascular disease, and kidney disease are interrelated clinical conditions. Diabetes is the primary contributor to CKD and eventual kidney failure in adults, increasing the risk of cardiovascular complications and mortality. Cardiovascular disease is emerging as the leading cause of death in people with CKD. In renal health, decreased estimated glomerular filtration rate (eGFR) is associated with increased mortality.3,4
Pediatric patients with CKD are at increased risk for cardiovascular complications such as arterial hypertension, dyslipidemia, increased arterial stiffness, and structural heart disease. These complications may result from underlying renal dysfunction and the complex interplay between traditional and CKD-specific risk factors.3,4 Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are a drug class originally developed to lower blood glucose levels in diabetes.5) However, recent studies have shown additional benefits. Prominent landmark trials of SGLT2i have demonstrated their cardiovascular benefits, including a reduction in major adverse cardiovascular events (such as heart attack, stroke, and cardiovascular death), fewer hospitalizations for heart failure, and a lower risk of all-cause death. In addition, these studies have shown significant renal health benefits, delaying the onset of dialysis, regardless of the patient's diabetes status.6-9) The consistent and remarkable cardiorenal benefits observed in these large trials have led to the rapid inclusion of SGLT2i therapy not only in diabetes guidelines, but also in cardiovascular and renal guidelines.5
This review provides a critical synthesis of the existing scientific evidence on SGLT2 inhibitors in pediatric populations with kidney diseases, providing insights into their mechanism of action, clinical efficacy, and potential benefits and implications in this patient population.
SGLT2i mechanism of action
The SGLT2 protein is a member of to the sodium substrate symporter gene family (SSSF), which comprises sodium-glucose symporter families encoded by the SLC5A gene. It acts as a glucose reabsorber in the renal cortex, and inhibition of this cotransporter can increase glucose excretion and correct hyperglycemia.10
Phloridzin, the first SGLT inhibitor derived from apple trees, inhibited renal glucose reabsorption. Despite the limitations of early derivatives such as T-1095, later gliflozins (dapagliflozin, empagliflozin, canagliflozin) overcame toxicity and poor selectivity, gaining approval for effective SGLT2 inhibition.10-12
SGLT2i, or gliflozins, have transformed patient care and revolutionized the treatment of diabetes, cardiovascular disease, and CKD, with significant beneficial effects on cardiovascular and renal protection.10-12
The origin of these drugs dates back to 2012, when the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) officially approved the use of SGLT2i to lower blood glucose in people with type 2 diabetes mellitus (T2DM; Table 1).10-13
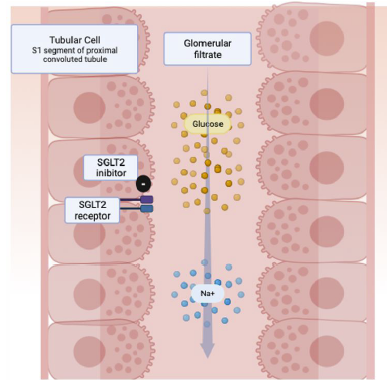
SGLT2 reabsorbs most of the filtered glucose primarily in the first and second proximal tubule segments, in contrast to SGLT1, which manages low glucose concentrations in the third segment. SGLT1 is particularly abundant in enterocytes and mediates intestinal glucose uptake. High selectivity in inhibiting SGLT2 is preferred to avoid interfering with glucose absorption in the intestine.12,13
The hyperglycemia characteristic of diabetes leads to increased glucose filtration from the glomeruli into the proximal tubules, where increased amounts are subsequently reabsorbed. This process is associated with compensatory upregulation of SGLT2 and SGLT1 expression.14,15 SGLT2i contribute to increased glucosuria by competitively inhibiting renal glucose reabsorption. They prioritize cotransporters over glucose, demonstrating greater affinity at the luminal surface for prolonged residence time. 14,15
SGLT2i effectively lower the renal threshold for glucose, but their glucosuric effect is self-limiting, minimizing the risk of clinically significant hypoglycemia. This self-limiting mechanism is a result of the dual action of the inhibitor: increasing glucosuria while lowering blood glucose levels.14,15
SGLT2i induce natriuresis, resulting in a 7% reduction in plasma volume and an average blood pressure reduction of 4/2 mm Hg. In addition to reducing glucose-induced Na+ entry into cells, they also inhibit Na+ entry through Na+-H1 antiporter 3 (NHE3) activity. In rodent models, SGLT2 and NHE3 colocalization in the proximal tubule brush border membrane suggests that inhibition of SGLT2 also reduces NHE3 activity.12-15
The functional link between NHE3 and SGLT2i may involve membrane-associated proteins, such as the 17-kD protein, maintaining transporter proximity. Despite reducing bicarbonate reabsorption, SGLT2i prevent metabolic acidosis by significantly increasing ammoniagenesis. In advanced stages of CKD, the blood pressure-lowering effect persists, which cannot be explained by Na+ excretion alone, as the natriuretic effect declines below an eGFR of 45 mL/min.12-15
SGLT2i exhibit antihypertensive effects through beneficial effects on arterial stiffness, weight loss, and reduced sympathetic nerve activity. Despite lowering plasma volume and blood pressure, there is no compensatory increase in heart rate, indicating a dampening effect on sympathetic nerve activity. Studies support a crosstalk between sympathetic nerve activity and SGLT2i, suggesting that the reduction in sympathetic tone may contribute to cardiovascular benefits beyond glycemic control (Figure 1).12-15
Table 1 List of main SGLT2 inhibitors
| Agent | Dose (mg/day) |
| Dapagliflozin | 5 - 10 |
| Canagliflozin | 100 - 300 |
| Empagliflozin | 10 - 25 |
| Ertugliflozin | 5 - 15 |
| Sotagliflozin | 200 |
| Ipragliflozin | 25 - 50 |
| Luseogliflozin | 2.5 - 5 |
| Tofogliflozin | 20 - 40 |
Diabetic kidney disease
All forms of diabetes increase the risk of impaired kidney function. Conventional treatments, which focus on blood pressure control and glucose management, slow but do not stop diabetic kidney disease (DKD). Growing evidence suggests that SGLT2i independently and additively protect against the onset and progression of DKD, complementing renin-angiotensin-aldosterone system (RAAS) blockade.16,17
Recent studies have consistently demonstrated the efficacy of SGLT2i in achieving glycemic control in patients with T2DM.18,19
SGLT2i are consistently effective regardless of insulin status, i.e., insulin resistance or deficiency. Their ability to lower glucose levels is particularly strong in individuals with elevated blood glucose concentrations, making them valuable in controlling prandial glucose excursions.18,19
Meta-analyses examining the reduction in glycated hemoglobin (HbA1c) levels with SGLT2i in T2DM consistently report reductions of approximately 0.5 to 1% (6-11 mmol/mol) from a baseline of approximately 8% (64 mmol/mol). The DURATION 8 trial compared dapagliflozin with exenatide (a GLP-1 agonist) in 695 patients with T2DM who were experiencing difficulties with glycemic control. Patients were assigned to receive dapagliflozin, exenatide, or a combination of dapagliflozin plus exenatide. Similar reductions in HbA1c were observed in patients receiving dapagliflozin and exenatide alone, with dapagliflozin also leading to greater reductions in body weight. The combination of exenatide and dapagliflozin not only improved glycemic control but also reduced cardiovascular risk factors and was well tolerated.20) The SUSTAIN 8 trial compared the GLP-1 agonist semaglutide subcutaneously with canagliflozin in 788 patients with uncontrolled type 2 diabetes and showed superiority of semaglutide over canagliflozin in both body weight and HbA1c reduction.21) The PIONEER 2 trial compared oral semaglutide with empagliflozin in patients with uncontrolled T2DM, with semaglutide showing a greater reduction in HbA1c but a similar result in weight loss compared with empagliflozin.22
Because of their unique mechanism of action compared with other glucose-lowering agents, SGLT2i can be combined with several agents, including insulin. In addition, they often lead to a reduction in the required insulin dose. However, it is important to note that SGLT2i cannot replace the need for adequate insulin to maintain basic metabolic functions.20-22
Several meta-analyses of renal data from the above cardiovascular outcomes trials and other studies have consistently confirmed that SGLT2i reduce a composite of worsening eGFR, ESKD, or renal death by approximately 33%.23,24
In the EMPA-REG OUTCOME trial, the composite renal outcome was significantly reduced by 46% in the empagliflozin groups. After the initial decline in eGFR, the use of empagliflozin was associated with a slight annual decline in eGFR (0.19 ± 0.11 mL/min/1.73m2/year), in contrast to a more rapid decline observed in the placebo group (1.67 ± 0.13 mL/min/1.73m2/year). The progression to macroalbuminuria (urine albumin-creatinine ratio [UACR] >300 mg/g) was reduced by 38% with empagliflozin, which was associated with a significant reduction in the number of patients who experienced a doubling of serum creatinine, a decline in eGFR to ≤45 mL/min/1.73m2, and initiation of renal replacement therapy.23,24
In the DECLARE-TIMI 58 trial, the use of dapagliflozin was associated with a 47% reduction in a renal composite of a sustained decline in eGFR of ≥ 40% to < 60 mL/min/1.73m2, new ESKD, or death from a renal cause. The eGFR decline (by ≥ 40% to < 60 mL/min/1.73m2) was 46% less with dapagliflozin, and there were also significant reductions in ESKD and renal death. In addition, dapagliflozin reduced new-onset albuminuria by 21% and new-onset macroalbuminuria by 46%.25,26
In the CANVAS trials, canagliflozin was associated with a 40% reduction in a renal composite of sustained (≥ 2 consecutive measurements) reduction (≥ 40%) in eGFR, need for renal replacement therapy, or death from renal causes. Progression of albuminuria was reduced by 27% with canagliflozin, and many patients who received canagliflozin showed a reduction in micro or macroalbuminuria.27
Nevertheless, it should be noted that these studies included a relatively small number of patients with advanced CKD, such as those with eGFR < 45 mL/min/1.73 m2 or advanced macroalbuminuria.
To address this gap, the CREDENCE trial intentionally recruited patients with T2DM with an eGFR range of 30-90 mL/min/1.73m2, UACR > 300 to < 5,000 mg/g, and angiotensin receptor blocker (ARB) therapy. The results showed a 30% lower incidence of the renal composite outcome with canagliflozin. Specifically, the rate of eGFR decline was significantly reduced in the canagliflozin group compared to the placebo group, and by year five, the rate of eGFR decline was markedly lower (by 2.6 mL/min/1.73m2). Canagliflozin also demonstrated a 32% reduction in ESKD cases and a 34% reduction in renal deaths. The drug effectively reduced UACR by 31% at six months, and more patients experienced a 30% reduction in UACR. The efficacy of SGLT2i in slowing eGFR decline and reducing albuminuria progression was consistent in patients with baseline eGFR > or < 45 mL/min/1.73m2 and UACR > or < 1,000 mg/g. SGLT2i demonstrated efficacy in slowing eGFR decline in patients with a baseline eGFR < 30 mL/min/1.73m2, and the effect of SGLT2i on these parameters remained independent of glycemic status, type of RAAS blockade, and presence of atherosclerotic cardiovascular disease. Secondary outcomes, such as cardiovascular death or hospitalization for heart failure, were also significantly reduced with canagliflozin (hazard ratio [HR] 0.69; 95% confidence interval [CI] 0.57-0.83; p <0.001). The CREDENCE study showed that canagliflozin provides broad cardiovascular and renal protection in patients with T2DM and CKD. Importantly, the rates of adverse events were similar between the canagliflozin and placebo groups, suggesting a favorable safety profile for the drug.28,29
Kidney disease
Beyond glycemic control, SGLT2i have also emerged in recent years as promising agents in the context of renal disease and cardiometabolic benefits in adults.30
Randomized clinical trials and observational studies primarily in individuals diagnosed with T2DM have consistently shown that SGLT2i can effectively slow eGFR decline. In addition, these studies have suggested that SGLT2i may contribute to a reduction in the incidence of microalbuminuria and have the potential to prevent or even reverse the progression of proteinuria.31,32
Most research on SGLT2i in kidney disease has been conducted in DKD. By modulating renal hemodynamics and reducing intraglomerular pressure, SGLT2i play a pivotal role in slowing the progression of kidney damage.31,32
The DAPA-CKD study evaluated the effect of dapagliflozin in people with (67%) and without (33%) T2DM with renal impairment (eGFR 25-75 mL/min/1.73m2, mean 43.1 mL/min/1.73m2; UACR 200-5000 mg/g, median 950 mg/g, 48.3% of patients with UACR > 1000 mg/g). The standard of care for all patients included RAAS blockade. Dapagliflozin demonstrated a significant reduction in the primary outcome of decline in eGFR, ESKD, renal mortality, or cardiovascular mortality compared to placebo (HR 0.61; 95% CI 0.51-0.72; p <0.001). Dapagliflozin also reduced hospitalization for heart failure and cardiovascular mortality (HR 0.71; 95% CI 0.55-0.92; p=0.009). Notably, these benefits extended to patients with and without diabetes. The incidence of adverse events was similar in the dapagliflozin and placebo groups.33
The DAPA-HF and EMPEROR-REDUCED trials, which investigated dapagliflozin and empagliflozin, respectively, included patients with and without diabetes, all diagnosed with heart failure with reduced ejection fraction. While the primary focus of these trials was cardiovascular events, they also reported a lower rate of worsening of a renal composite outcome associated with SGLT2i. Notably, a slower rate of eGFR decline was observed in individuals with and without diabetes, and this effect was independent of baseline renal status.34-36
In the DAPA-HF trial, patients with an eGFR as low as 30 mL/min/1.73m2 (mean baseline eGFR of 66 mL/min/1.73 m2) experienced a numerical reduction (by 39%, although not statistically significant) in the renal composite outcome of ≥50% sustained decline in eGFR, ESKD, or renal death with dapagliflozin. However, the rate of eGFR decline was significantly lower with dapagliflozin compared to placebo.34-36
The EMPEROR-REDUCED trial included patients with an eGFR as low as 20 mL/min/1.73m2 (mean baseline eGFR of 62 mL/min/1.73m2) and showed that empagliflozin reduced the renal composite of sustained decline in eGFR, dialysis, or renal transplantation by 50%. Additionally, the rate of eGFR decline was significantly lower with empagliflozin compared to placebo (mean -0.55 versus -2.28 mL/min/1.73m2/year).37
The EMPA-KIDNEY trial specifically enrolled non-diabetic patients with renal impairment. The study’s primary outcome was the first occurrence of a composite of kidney disease progression, which included a sustained eGFR decline ≥40% from randomization, kidney replacement therapy, a sustained eGFR decline to <10 mL/min/1.73m2, and cardiovascular death. The study showed that empagliflozin had beneficial effects on renal function and cardiovascular mortality in patients with CKD (regardless of their diabetic status) who were already on adequate doses of angiotensin-converting enzyme inhibitors (ACEi)/ARBs.38
With the growing understanding that SGLT2i did not compromise renal safety and provided cardio-renal benefits, indications have expanded to include conditions such as focal segmental glomerulosclerosis (FSGS), immunoglobulin A (IgA) nephropathy, and CKD without diabetes. Recent results suggest potential benefits in slowing progression of kidney damage, reducing proteinuria, and preserving renal function in these populations. Further investigation is underway to elucidate the specific mechanisms and optimal use of SGLT2i in non-diabetic kidney disease.38
These findings have led to a paradigm shift in the management of DKD, placing SGLT2i as a cornerstone in the therapeutic armamentarium.39
The safety profile of SGLT2i in kidney disease appears to be favorable, with a low incidence of adverse events reported in clinical trials. However, healthcare providers must remain vigilant, particularly regarding the risk of volume depletion, urinary tract infections, and acute kidney injury. Choi et al. described the rate of adverse events with SGLT2i and showed that urinary tract infection had the highest incidence (62.1 events/1000 person-years), followed by genital mycotic infection (58.0 events/1000 person-years). The median time to occurrence of each adverse event was also investigated. Adverse events occurring earlier included genital mycotic infection (3.9 months) and hyperkalemia (5.5 months), and those occurring later included acute kidney injury (10.8 months) and bone fracture (19.1 months).39) Individual patient characteristics, including baseline renal function, comorbidities, and concomitant medications, should be carefully considered to ensure the judicious use of SGLT2i in this population.39
As the GFR decreases, the amount of glucose delivered to the proximal tubules decreases proportionally. Consequently, the glucosuric (and therefore the antihyperglycemic) effect of SGLT2i decreases approximately in proportion to the GFR decline. In recognition of this correlation, product labels for SGLT2i specify GFR values below which it is recommended not to initiate or continue treatment.40
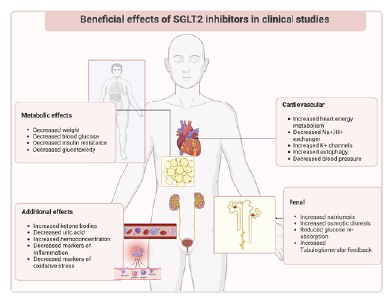
In summary, the multiple effects of SGLT2i on renal function include reducing hyperfiltration and inflammation, improving oxygen availability, and reducing oxidative stress. SGLT2i have shown promise in preserving glomerular viability through adenosine-mediated vasoconstriction, influencing sodium handling, altering nutrient metabolism, and reducing inflammation and fibrosis markers. These effects are not only related to their glycemic control properties, but also extend to cardiovascular benefits and prevention of heart failure. In addition, SGLT2i have been shown to be renoprotective independent of their effects on blood pressure and weight loss (Figure 2).
Cardiovascular disease and metabolic control
The year 2008 marked a pivotal moment when the U.S. Food and Drug Administration (FDA) mandated the inclusion of cardiovascular outcomes in diabetes trials. Subsequent cardiovascular outcomes studies have demonstrated additional benefits of SGLT2i.41
In addition to their aforementioned effects, SGLT2i have consistently shown in clinical trials to lower blood pressure specifically systolic blood pressure by 3-5 mmHg and diastolic blood pressure by 2-3 mmHg without inducing hypotension. In addition, these inhibitors consistently reduced the risk of new heart failure and worsening of existing heart failure. The observed benefits were seen within a few weeks of starting an SGLT2i, in people with and without diabetes, and remained independent of the magnitude of the effects on glucose, weight, or blood pressure.41
Several clinical trials have evaluated the impact of these drugs on cardiovascular outcomes. Key cardiovascular benefits associated with SGLT2i have bee reported in trials such as EMPA-REG OUTCOME (empagliflozin), CANVAS Program (canagliflozin), and DECLARE-TIMI 58 (dapagliflozin), which demonstrated a reduction in major adverse cardiovascular events, including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke.42
The EMPA-REG OUTCOME trial is the earliest cardiovascular outcomes trial for SGLT2i and demonstrated significant cardiovascular benefits. The study included 7020 patients with T2DM who were randomized to receive either empagliflozin or placebo. The empagliflozin group showed a reduction in major adverse cardiovascular events, including myocardial infarction, stroke, and cardiovascular death, and a reduction in hospitalizations for heart failure. This landmark study was the first to show positive cardiovascular outcomes in patients with T2DM.23,24
The CANVAS Program trial compared canagliflozin with placebo in 10,142 patients with T2DM. The canagliflozin group showed a notable reduction in major adverse cardiovascular events. Furthermore, there was a reduction in hospitalizations for heart failure and cardiovascular death in the canagliflozin group. However, an increased risk of amputation, particularly of the toe or metatarsal, was observed in patients receiving canagliflozin.23,24
The DECLARE-TIMI 58 trial investigated dapagliflozin versus placebo in 17,160 patients with T2DM and showed a reduction in heart failure-related death and hospitalization. Notably, dapagliflozin did not significantly reduce the rate of major adverse cardiovascular events. Renal events were less common with dapagliflozin (HR 0.76; 95% CI 0.67-0.87).25,26
A meta-analysis conducted by the TIMI Study Group that included data from the EMPA-REG OUTCOME, CANVAS Program, and DECLARE-TIMI 58 trials in a total of 34,322 patients showed that SGLT2i reduced hospitalizations for heart failure (HR 0.77; 95% CI 0.71-0.84; p <0.0001) and slowed progression of kidney disease (HR 0.55; 95% CI 0.48-0.64, p <0.0001) in patients with and without cardiovascular disease or a history of heart failure.42
With respect to benefits in heart failure, SGLT2i have demonstrated significant benefits in reducing the risk of heart failure events, as observed in studies such as EMPEROR-REDUCED (Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Reduced Ejection Fraction) and DAPA-HF (Dapagliflozin and Prevention of Adverse Outcomes in Heart Failure).34-36
In addition, some studies have shown reductions in measures of atherosclerotic cardiovascular disease (such as cardiovascular death, nonfatal myocardial infarction, and stroke) with SGLT2i (Table 2).43-47
Improvements in lipid profile and modulation of body weight are among the effects observed, suggesting a broader impact of these agents on metabolic health. In clinical trials in patients with T2DM, the weight loss effect of SGLT2i has typically been approximately 3 kg, with stabilization at 6-12 months. Weight loss is generally attributed to calorie loss via glucosuria, which reduces fat mass. It is worth noting that a concomitant reduction in plasma volume may also contribute to the observed weight loss.43-47
Table 2 Major randomized controlled trials of SGLT2i and their main results
| RCT | Population characteristics | Main CV outcomes | Main renal outcomes |
| EMPA-REG OUTCOME | Type 2 diabetes | 34% reduction (HR 0.83, 95% CI 0.74-0.99; p=0.04) in the composite of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke | 39% reduction (HR 0.61, 95% CI 0.53-0.70; p <0.001) in the renal-specific composite outcome * |
| CANVAS | Type 2 diabetes | 22% reduction (HR 0.86, 95% CI 0.75-0.97; p=0.02) in the composite of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke | 40% reduction (HR 0.60, 95% CI 0.47-0.77; p <0.001) in the renal-specific composite outcome * |
| DECLARE-TIMI 58 | Type 2 diabetes | 17% reduction (HR 0.83, 95% CI 0.73-0.95; p=0.005) in the composite of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke | 47% reduction (HR 0.53, 95% CI 0.43-0.66; p <0.0001) in the renal-specific composite outcome * |
| DAPA-HF | Patients with chronic HFpEF and NYHA II-IV | 26% reduction (HR 0.74, 95% CI 0.65-0.85) in the composite of cardiovascular death or HHF | 26% reduction (HR 0.71, 95% CI 0.44-1.16; p=0.17) in the renal-specific composite outcome * |
| EMPEROR-REDUCED | Patients with chronic HFpEF and NYHA II-IV | 25% reduction (HR 0.75, 95% CI 0.65-0.86; p <0.001) in the composite of cardiovascular death or HHF | 50% reduction (HR 0.50, 95% CI 0.32-0.77) in the renal-specific composite outcome * |
| EMPEROR-PRESERVED | Patients with chronic HFpEF and NYHA II-IV | 21% reduction (HR 0.79, 95% CI 0.69-0.90; p <0.001) in the composite of cardiovascular death or HHF | Improvement in the eGFR slope (+1.36 mL/min/1.73 m2per year, p <0.001) |
| CREDENCE | Type 2 diabetes | 31% reduction (HR 0.69, 95% CI 0.57-0.83; p <0.001) in the composite of cardiovascular death or HHF | 34% reduction (HR 0.66, 95% CI 0.53-0.81; p <0.001) in the renal-specific composite outcome * |
| DAPA-CKD | Inclusion criteria: eGFR 25-75 mL/min/1.73 m2 Evidence of increased albuminuria and UACR 200-5000 mg/g | 29% reduction (HR 0.71, 95% CI 0.55-0.92) in the composite of cardiovascular death or HHF | 39% reduction (HR 0.56, 95% CI 0.45-0.68) in eGFR of at least 50%, ESKD, or renal or cardiovascular death |
| EMPA KIDNEY | Established CKD: eGFR 20-44 mL/min/1.73 m2or 45-90 mL/min/1.73 m2with UACR ≥ 200 mg/g | 28% reduction in primary outcomes of kidney disease progression, sustained decline in eGFR ≥40 mg/dL, or renal or cardiovascular death (HR 0.72, 95% CI 0.64-0.82; p <0.001) |
CI, confidence interval; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; ESKD, end-stage kidney disease; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HHF, hospitalization for heart failure; HR, hazard ratio; NYHA, New York Heart Association; RCT, randomized controlled trial; UACR, urine albumin-creatinine ratio. * Composite outcome - doubling of serum creatinine, end-stage kidney disease (ESKD), renal death, or cardiovascular death
SGLT2i in the pediatric population
In the pediatric population, studies suggest a potential reduction in glycemic control with SGLTi, mirroring findings in the adult population.
The DINAMO study was a double-blind, placebo-controlled trial conducted at 108 centers in 15 countries in patients with T2DM aged 10-17 years, HbA1c 6.5-10.5% (48-91 mmol/mol), previously treated with metformin or insulin. Patients were randomly assigned (1:1:1) to receive either empagliflozin 10 mg, oral linagliptin (dipeptidyl peptidase-4 [DPP-4] inhibitor) 5 mg, or placebo. Participants in the empagliflozin group with HbA1c greater than 7.0% (<53 mmol/mol) at week 12 underwent a second double-blind randomization (1:1) at week 14 to either stay on 10 mg or increase to 25 mg. At week 26, participants in the placebo group were randomly reassigned (1:1:1) in a double-blinded manner to receive linagliptin 5 mg or one of the empagliflozin doses (10 mg or 25 mg). The primary outcome was the change from baseline in HbA1c at week 26. Safety assessments were conducted through week 52. The analysis showed a significant reduction of -0.84% [-9.2 mmol/mol] in the pooled empagliflozin group versus placebo. The corresponding change from baseline for linagliptin versus placebo was -0.34%. A secondary endpoint showed that empagliflozin reduced fasting plasma glucose at week 26, (-35.2 mg; p=0.0035). Adverse events occurred in 34 (64%) participants in the placebo group, 40 (77%) in the pooled empagliflozin group, and 37 (71%) in the linagliptin group through week 26. Severe adverse events were reported in two participants (4%) in the placebo group, one (2%) in the pooled empagliflozin group, and one (2%) in the linagliptin group. Hypoglycemia was the most frequently reported adverse event, with higher rates in the active drug groups compared with placebo. No cases of severe hypoglycemia were reported.48
In June 2023, the U.S. FDA approved empagliflozin to reduce blood glucose levels in addition to diet and exercise in children with T2M aged 10 years and older based on the results of the phase III DINAMO trial.48
T2NOW was a randomized, double-blind, placebo-controlled phase III trial designed to assess the efficacy and safety of dapagliflozin as adjunctive therapy in children and adolescents with T2DM who were already receiving metformin, insulin, or a combination of both. Participants were randomly assigned to receive dapagliflozin, saxagliptin, or placebo. Those on an active drug underwent additional randomization to either maintain their current dose or escalate to a higher dose of the same treatment. The study primary endpoint was the change in HbA1c at 26 weeks. Dapagliflozin was administered at 5 or 10 mg doses and saxagliptin at 2.5 or 5 mg doses. Secondary endpoints included changes in fasting plasma glucose and the proportion of patients (with A1c ≥7% at baseline) achieving A1c <7.0% (53 mmol/mol) at 26 weeks. The study results showed a substantial reduction in A1c, a key indicator of average blood glucose levels, in patients treated with dapagliflozin compared to placebo. The adjusted mean change in A1c was -0.62% for dapagliflozin and +0.41% for placebo, representing a significant difference of -1.03%. Statistical significance was achieved not only for the primary endpoint, but also for all secondary endpoints at week 26. These results suggested that dapagliflozin has the potential to provide clinically meaningful improvements in glycemic control in children and adolescents with T2DM. In addition, the safety results were consistent with those observed in adults with T2DM and with the well-established safety profile of dapagliflozin.49
Following these results, the European Medicines Agency (EMA) approved dapagliflozin in children with T2DM aged 10 years and older.49
In the study by J Liu and colleagues, 9 patients with renal disease received dapagliflozin 5 mg per day (body weight ≤30 kg) or 10 mg per day (body weight >30 kg) for 12 weeks.50The primary renal diagnoses were Alport syndrome (n=5), Dent disease (n=1), and proteinuria (n=3). One patient was lost to follow-up during the first four weeks. After 12 weeks of treatment, eight patients had a reduction in 24-hour proteinuria (range 0.02-1.90 g/m2). Dapagliflozin treatment was associated with a significant reduction in 24-hour proteinuria at 4 and 12 weeks compared to baseline (1.4 [0.9-2.1] vs 2.1 [1.4-2.6] g/m2; 1.5 [1.2-2.2] vs 2.1 [1.4-2.6] g/m2; p <0.05, respectively). The percentage change in 24-hour proteinuria from baseline showed a reduction of 33.3% (95% CI 23.1-43.4) at four weeks and 22.6% (95% CI 8.3-36.9) at 12 weeks of treatment. While there was no significant change in plasma albumin levels at four weeks, a significant increase was observed at 12 weeks compared to baseline (37.5 ± 7.9 vs 35.3 ± 6.7 g/l, p <0.05). No significant changes in eGFR were observed at week 4, although a slight decrease was observed at week 12 compared to baseline (103.8 ± 28.2 vs 109.2 ± 32.0 mL/min/1.73 m2, p=0.048). Body mass index remained stable throughout the 12 weeks of treatment. No patient discontinued dapagliflozin due to adverse events. In this study, dapagliflozin showed efficacy in the treatment of children with inherited proteinuric CKD with eGFR greater than 60 mL/min/1.73m2, suggesting that it may be a novel alternative for the management of proteinuric CKD in children, particularly in cases of monogenetic kidney disease.50
Ongoing studies in pediatric populations also suggest that these benefits may extend their therapeutic potential, opening the door to exploring the use of SGLT2i in pediatric patients with obesity and other cardiometabolic risk factors.48-50
Recent studies are also exploring the use of SGLT2i beyond diabetes, unveiling potential applications in conditions such as polycystic ovarian syndrome and non-alcoholic fatty liver disease in pediatric patients. Although these novel therapeutic options warrant further investigation, they suggest a broader impact of SGLT2i on pediatric endocrinology and metabolism.51,52
Even in the absence of comprehensive data on pediatric heart failure, pediatric centers have begun to incorporate SGLT2i in the treatment of children with heart failure. In their study, Butts and colleagues surveyed several institutions and outlined current trends and practices regarding the use of SGLT2i in pediatric heart failure. The participating institutions provided insights into the characteristics of this patient population, the selection of specific drugs, and protocols for laboratory monitoring of patients undergoing treatment with SGLT2i. Of 29 institutions, 18 (62%) confirmed the use of SGLT2i. Of the 11 institutions that that had not yet implemented SGLT2i, two planned to do so in the next 0-3 months, five in the next 3-6 months, three in more than 6 months, and only one had no current plans to implement SGLT2i. In the 18 institutions that had implemented SGLT2i, approximately 185 patients had been treated with these agents. The median lower age of patients prescribed SGLT2i was 12.5 years (interquartile range [IQR] 9.5-15) and the median upper age was 20.5 years (IQR 17.8-24.3). Each institution had a median of four patients treated with these agents (IQR 3-7), and the highest estimated number of patients treated at a single institution was 70. Regarding specific SGLT2i, 13 institutions (72%) reported the use of dapagliflozin, while 10 (55.6%) reported the use of empagliflozin. SGLT2i have been rapidly incorporated into the protocols of several pediatric heart failure centers, typically as 4th-line agents. However, there is notable variability in practice patterns among centers, highlighting the need for additional studies aimed at establishing best practices in the use of SGLT2i in the treatment of pediatric heart failure.53
The limited pediatric data prompts extrapolation of evidence from adults for the potential use of SGLT2i in pediatric glomerulopathies and CKD, raising concerns about side effects. Pediatric-specific considerations include increased severity of urogenital infections and potential complications in immunosuppressed patients. Despite these considerations, nephrologists are considering expanding the use of SGLT2i to pediatric populations, inspired by their success in adults. These inhibitors, tested in adult proteinuric glomerulopathies, such as IgA nephropathy, have shown efficacy in slowing kidney disease progression. The complex genetics of children with glomerulopathies, influenced by low-effect genetic polymorphisms, contributes to their predisposition to CKD progression. Pediatric nephrologists, aware of the side effects of immunosuppressive therapy, are rethinking its use and focusing on optimizing antiproteinuric treatments and risk reduction. SGLT2i, with their physiological aspects, offer potential applicability to all proteinuric diseases in children. As an adjunct to RAAS inhibitors, SGLT2i may become valuable options in pediatric CKD to manage metabolic complications associated with disease progression, supported by the positive experience with antiproteinuric therapy in children with glomerular disease.54
Conclusion
In conclusion, the exploration of SGLT2i in pediatric healthcare represents a promising frontier for the integration of these agents into clinical practice, with potential benefits in glycemic control, cardiometabolic health, and renal function, underscoring the multifaceted potential of SGLT2i in pediatric healthcare.51,52) While challenges and questions remain, ongoing research is gradually illuminating the way forward. However, cautious consideration of safety concerns and the need for long-term and well-controlled clinical trial data are imperative as the scientific community navigates this evolving landscape, paving the way for informed and evidence-based pediatric therapeutic strategies. As our understanding deepens, SGLT2i may become valuable tools in the pediatric clinician’s armamentarium, offering innovative solutions to improve the health and well-being of children facing a spectrum of metabolic and renal challenges. Future directions include expanding research to additional pediatric populations, refining dosing strategies, and elucidating the long-term effects on growth and development.
Authorship
Joana Freitas - Conception and design; Data analysis and interpretation; Article writing
Sara Nogueira Machado - Substantive scientific and intellectual contributions to the study;
Beatriz Vieira - Substantive scientific and intellectual contributions to the study;
Sara Monteiro - Substantive scientific and intellectual contributions to the study;
Sameiro Faria - Substantive scientific and intellectual contributions to the study; Conception and design; Critical revision; Final approval
Teresa Costa - Substantive scientific and intellectual contributions to the study; Conception and design; Critical revision; Final approval
Conceição Mota - Substantive scientific and intellectual contributions to the study; Conception and design; Critical revision; Final approval