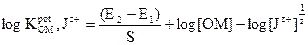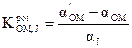Introduction
Oxymetazoline hydrochloride (OMCl), phenol, 3-[4,5-dihydro-1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethylmonohydrochloride [2315-02-8] (Scheme 1) is an adrenergic (nasal) drug [1].
A review of the literature revealed that several methods have been used for oxymetaoline hydrochloride determination. These include high performance liquid chromatography (HPLC) [2-13], gas chromatography [14], a two phase titration method [15], flow injection chemiluminometry [16,17], spectrophotometry [18-33] and spectrofluorimetry [34]. A plastic PVC membrane ion selective electrode method, based on oxymetazoline-phosphotungstate, has been reported [35]. Carbon paste electrodes (CPEs) have attracted attention as ion selective electrodes, mainly due to their advantages over membrane electrodes, such as renewability, stable response, low ohmic resistance and no need of an internal solution [36]. The aim of this work is to describe the construction and performance characteristics of oxymetazoline modified carbon paste electrodes based on oxymetazoline-tetraphenylborate and oxymetazoline-reineckate as electroactive materials, using dibutyl phthalate and dioctyl phthalate as solvent mediators, respectively. The developed carbon paste selective electrodes were used for OMCl determination in Afrin nasal drops pharmaceutical preparation (0.05%).
Experimental
Reagents and materials
All chemicals were of analytical grade. Double distilled water was used throughout all experiments. Pure grade oxymetazoline hydrochloride and the Afrin nasal drops (0.05%) pharmaceutical preparation were provided by Medical Union Pharmaceuticals Co., Abou Sultan, Egypt. Sodium tetraphenylborate (NaTPB), ammonium reineckate, dioctyl sebacate (DOS) and tricresyl phosphate (TCP) were obtained from Fluka. Dibutyl phthalate (DBP) and dioctyl phthalate (DOP) were obtained from Merck. Graphite powder (1-2 micron) was obtained from Aldrich Co.
Apparatus
Potentiometric and pH measurements were carried out using a Seibold G-103 digital pH/mV meter (Vienna, Austria). A C-100 Techne circulator thermostat model was used to control the test solutions temperature. A saturated calomel electrode (SCE) was used as the reference electrode. The electrode electrochemical system may be represented as follows: OM-carbon paste electrode / test solution // KCl salt bridge // saturated calomel electrode.
Ion pair preparation
The ion pairs, OM-TPB and OM-reineckate, were prepared by mixing a 100 mL of a 10-2 M OMCl solution with 100 mL of 10-2 M of sodium tetraphenyl borate or ammonium reineckate. The formed precipitates were filtered, washed thoroughly with bidistilled water and dried at room temperature. The composition of the ion pair was found to be 1:1, both for OM-TPB and OM-reineckate, as confirmed by elemental analysis data done at Microanalytical Center, Faculty of Science, Cairo University. The found percentage values were 82.54, 7.40 and 4.73, and the calculated values were 82.89, 7.65 and 4.83, for C, H and N, respectively, in the OM-TPB case, while in the OM-reineckate case, the found percentage values were 40.56, 4.93 and 18.62, and the calculated were 40.25, 5.40 and 18.77, for C, H and N, respectively.
Electrode assembly
Carbon paste was prepared by mixing the required amount of the ion pair with graphite powder (Aldrich, 1-2 micron) and dibutyl phthalate (DBP) or dioctyl phthalate (DOP) with a pasting liquid (the ratio of graphite powder to pasting liquid was 1:1) in a mortar, until it was uniformly wetted. The carbon paste electrode was prepared by successive packing of the carbon paste into the tip end of a home-made Teflon holder (2 mm), and electrical contact was achieved by a stainless steel rod (2 mm) connecting the paste to the mV meter.
Electrodes selectivity
The electrodes selectivity coefficients towards different Jz+ cationic species were determined by a separate solution method [37], in which the Nicolsky Eisenman equation was used:
where E1 and E2 are the electrode potentials in 1 x 10-3 mol L-1 OMCl and Jz+ interfering ions, respectively, and S is the slope in mV. In case of ions without charges, the selectivity coefficients were determined by the matched potential method (MPM) [38]. In this method, a known activity of the oxymetazoline ion solution was added into a reference solution containing a fixed activity of oxymetazoline ion, αOM (α'OM - αOM is the change in activity), and the corresponding potential change (ΔE) was recorded. Then, a solution of the interfering ion was added to the reference solution, until the same potential change (ΔE) was reached. The change in potential produced at the constant background of the primary ion must be the same in both cases:
where αj is the added interferent activity.
OMCl potentiometric determination
OMCl has been potentiometrically determined using the investigated electrodes by the standard addition method [39] and by potentiometric titration with a standard solution of NaTPB.
OMCl determination in Afrin nasal drops (0.05%)
The request amount of nasal drop solution (0.05%) was transferred to a separating funnel, 25 mL of a saturated solution of sodium borate were added and extracted with four 25 mL portions of chloroform, and then the extracts were combined in a second separating funnel. The combined chloroform extracts were extracted with two 20 mL portions of dilute hydrochloric acid (20 fold dilution), the acid extracts were combined in a 50 mL volumetric flask, and dilute hydrochloric acid was added to the mark and mixed. The contents of the measuring flask were transferred to a 100 mL beaker, and subjected to OMCl potentiometric determination, by using the standard addition method, as previously described in the pure drug solution case.
Results and discussion
Carbon paste electrodes composition
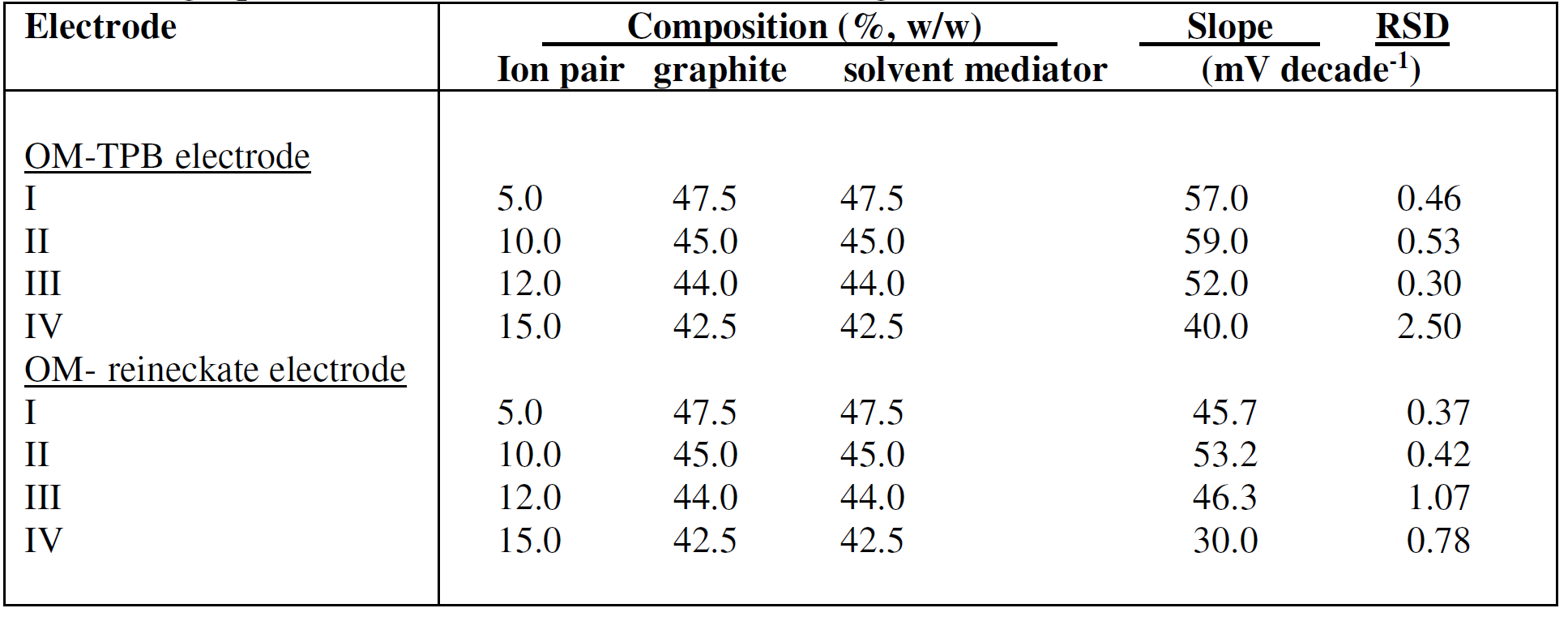
Four compositions containing the OM-TPB or OM- reineckate ion pairs, in the ratio of 5, 10, 12 and 15%, were prepared; the ratio of graphite to liquid mediators (DBP) was 1:1, after the electrodes soaking for 30 min in a 10-3 M OMCl solution (Table 1). Results indicated that the carbon paste electrode with an ion pair ratio of 10% exhibited the best performance characteristic, with slopes of 59.00 and 53.20 mV of concentration decade, at 25oC, for OM-TPB and OM-reineckate, respectively. The performance characteristics of the selective electrodes were primarily affected by the plasticizers, due to their effect on migration, ion pair formation and diffusion coefficient [40]. The plasticizers effect type on the OM electrodes performance characteristic was investigated by using four plasticizers with different polarities (DBP, DOP, DOS, and TCP), as shown in Table 2. The results indicate that DBP and DOP are the best solvent mediators for OM-TPB and OM-reineckate electrodes, respectively. Poor sensitivities for the electrodes using other plasticizers as liquid mediators are due to low distributions of the OM-TPB or OM- reineckate electroactive complexes in these solvents [41].
Table 1 Composition of oxymetazoline carbon paste electrodes and the slope of their calibration graphs, at 25 ± 1oC, and 30 min soaking in 1a 0-3 M OMCl solution.
Effect of pH
The effect of the solution pH on the electrode response was checked for two OMCl concentrations (1x10-4 and 1x10-3 M), by following the variation in potential with change in pH, by the addition of very small volumes of hydrochloric acid and sodium hydroxide (0.1-1.0 M). The results indicate that the investigated electrodes showed no pH response over the pH range from 2.0 to 9.4, for the two proposed electrodes. Fig. 1 represents potential versus pH profiles for OM-TPB electrode. The decrease in potential occurring at higher pH values is most probably attributed to the free oxymetazoline base formation in the solution, leading to a decrease in the oxymetazoline cation concentration (pKa = 10.6 [43]).
Table 2 Effect of solvent mediator on 10% OM-TPB and 10% OM-reineckate electrodes, and the slopes of their calibration graphs, at 25 ºC, and 30 min soaking in a 10-3 M OMCl solution.

Figure 1 pH effect of the test solution on the OM-TPB electrode potential response: (a) 1 x 10-4 M and (b) 1 x 10-3 M OMCl solutions.
Electrode life span
The life span of the proposed electrodes was investigated by periodically performing the calibration graphs, and calculating the response slope after the electrode was left in air without soaking in the drug; the electrode was preconditioned by soaking in 1x10-3 M OMCl, for 30 min, before use. The results indicate that, in the OM-TPB electrode case, the calibration graph slope remained constant, near 59.0 mV decade-1, for 1 day; then, it slightly decreased, reaching about 56.0, 55.0, 53.0, 52.0, 51.0 and 50.0 mV decade-1, after 8, 21, 30, 45, 60 and 90 days, respectively. In the OM-reineckate electrode case, the calibration graph slope remained constant near 58.0 mV decade-1, for 1 day; then, it slightly decreased, reaching about 52.0 and 50.0 mV decade-1, after 8 and 15 days, respectively. The response time of the proposed OM carbon paste electrodes was found to be < 10 seconds.
Effect of test solution temperature
To study the investigated electrodes thermal stability, calibration graphs were constructed at different test solution temperatures, and the isothermal coefficients (dE/dt) of these electrodes were calculated [44] to be 0.0010 and 0.0020 V/ ºC, for OM-TPB and OM-reineckate electrodes, respectively.
Selectivity
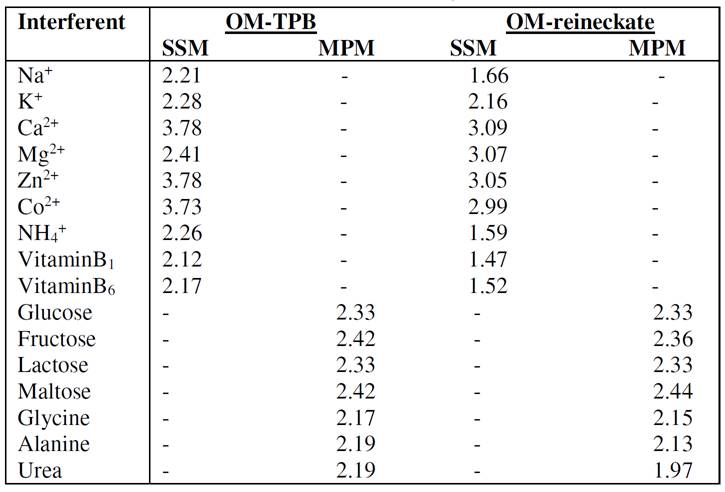
The influences of some inorganic cations, sugars, amino acids and vitamins, on the OM electrodes response, were investigated according to IUPAC recommendations, using the separate solution method (SSM) [37] or matched potential method [38]. The selectivity coefficients values, 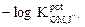 , presented in Table 3, indicate that the proposed carbon paste electrodes are highly selective towards the oxymetazoline cation. The inorganic cations do not interfere, because of the difference in their mobility and permeability, as compared to the oxymetazoline cation. In the sugars and amino acids case, the high selectivity is related to the difference in polarity and lipophilic nature of their molecules relative to the oxymetazoline cation.
, presented in Table 3, indicate that the proposed carbon paste electrodes are highly selective towards the oxymetazoline cation. The inorganic cations do not interfere, because of the difference in their mobility and permeability, as compared to the oxymetazoline cation. In the sugars and amino acids case, the high selectivity is related to the difference in polarity and lipophilic nature of their molecules relative to the oxymetazoline cation.
Analytical applications
The usefulness of the investigated electrodes was examined by using them for the OMCl potentiometric determination in pure solutions and in the Afrin nasal drops pharmaceutical preparation (0.05%). Standard addition and potentiometric titration methods were used in the pure solutions case. In the Afrin nasal drops pharmaceutical preparation case (0.05%), the standard addition method was used. The potentiometric titration method could not be used, because it had a lower OMCl concentration (0.5 mg / mL of the drops), and the potentiometric titration method needs a higher drug concentration. Fig. 2A and Fig. 2B represent the potentiometric titration methods using the OM-TPB electrode and OM-reineckate electrode, respectively.
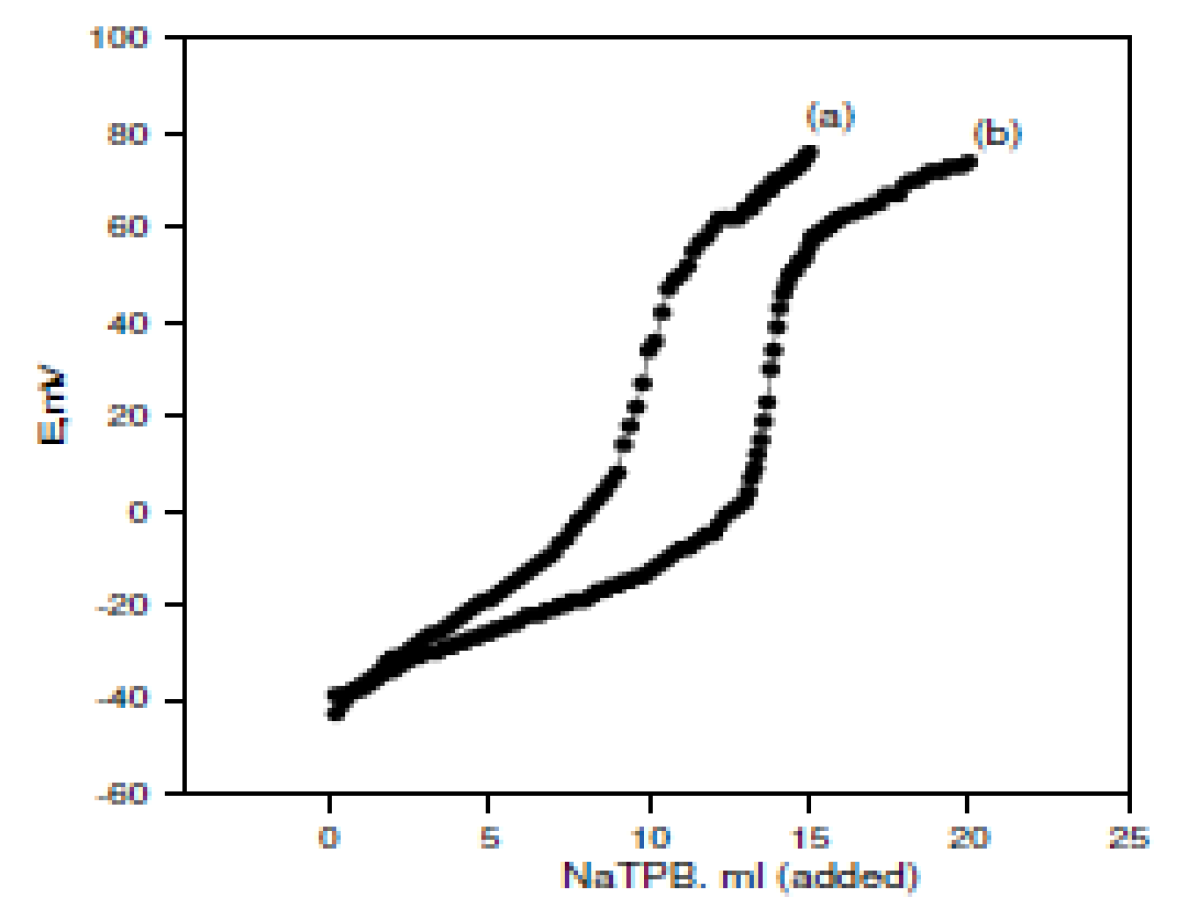
Figure 2A Potentiometric titrations of (a) 10 and (b) 15 mL of the 10-2M OMCl drug in a 10-2 M NaTPB solution using OM-TPB electrode.
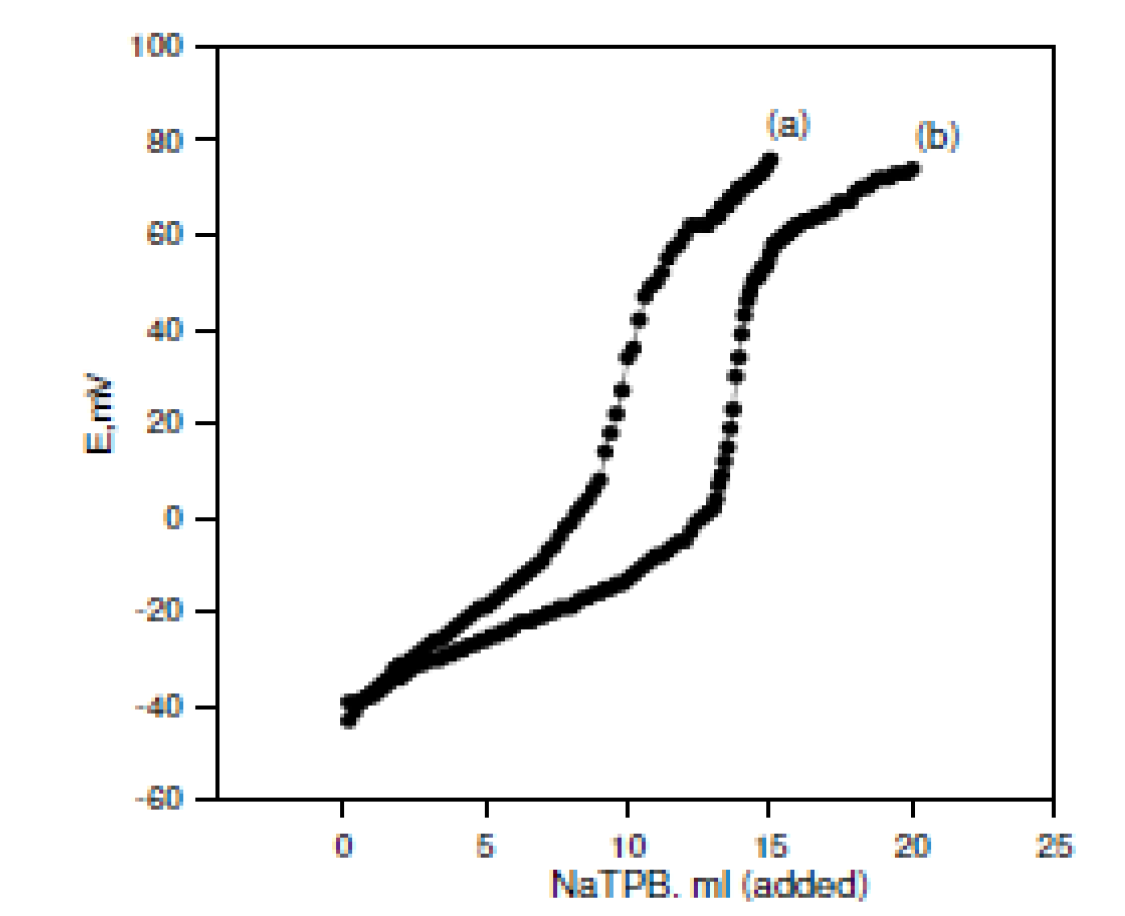
Figure 2B Potentiometric titrations of (a) 10 and (b) 15 mL of the 10-2M OMCl drug in a 10-2 M NaTPB solution using an OM-reineckate electrode.
The mean recovery and relative standard deviation values are briefed in Table 4.
The results were in good agreement with the label claims and with the values obtained using the official United States Pharmacopeia1 (Table 5). Statistical comparison of the accuracy and precision of the proposed methods with the official method was performed using Student's t- and F-ratio tests, at a 95% confidence level [45].
Table 4 OMCl determination by applying OM-TPB and OM-reineckate responsive electrodes.
| Sample | Standard addition method | Potentiometric titration method | ||||
|---|---|---|---|---|---|---|
| Taken (mg) | Mean recovery (%) | RSD (%) | Taken (mg) | Mean recovery (%) | RSD (%) | |
| OM-TPB | ||||||
| Pure solution | 1.84 | 99.11 | 0.70 | 22.9 | 99.40 | 0.45 |
| 2.30 | 98.40 | 0.62 | 34.5 | 98.40 | 0.55 | |
| 4.60 | 99.00 | 1.40 | ||||
| 11.50 | 98.90 | 0.88 | ||||
| Afrin nasal drops (0.05%) | 0.50 | 97.70 | 0.93 | |||
| 1.00 | 98.20 | 0.25 | ||||
| 1.50 | 99.11 | 0.12 | ||||
| 2.00 | 98.50 | 0.24 | ||||
| OM-reineckate | ||||||
| Pure solution | 1.84 | 97.70 | 1.11 | 22.9 | 99.60 | 0.50 |
| 2.30 | 98.50 | 1.22 | 34.5 | 98.30 | 0.32 | |
| 4.60 | 98.30 | 1.49 | ||||
| 11.50 | 97.90 | 1.22 | ||||
| Afrin nasal drops (0.05%) | 0.50 | 98.11 | 0.79 | |||
| 1.00 | 98.40 | 0.15 | ||||
| 1.50 | 99.40 | 0.45 | ||||
| 2.00 | 99.30 | 0.36 | ||||
Relative standard deviation (four determinations).
Table 5 Statistical comparison between the results of the analysis of an Afrin nasal drop pharmaceutical preparation (0.05%), applying the proposed standard addition method and the official method, using carbon paste electrode.
| Parameter | Standard addition method | Official method [1] |
|---|---|---|
| OM-TPB electrode | ||
| Mean Recovery % | 98.37 | 99.22 |
| SD | 0.378 | 0.598 |
| RSD | 0.385 | 0.603 |
| F-ratio | 2.45 (9.28)a | |
| t-test | 2.37 (2.447)b | |
| OM-reineckate electrode | ||
| Mean Recovery % | 98.80 | 99.43 |
| SD | 0.42 | 0.598 |
| RSD | 0.43 | 0.602 |
| F-ratio | 1.96 (9.28)a | |
| t-test | 1.70 (2.447)b |
SD: Standard deviation; RSD: Relative standard deviation; a Tabulated F-ratio value at 95% confidence level; b Tabulated t-value at 95% confidence level and six degrees of freedom.
Conclusions
The proposed oxymetazoline modified carbon paste electrodes based on OM-TPB and OM-Rein ion pairs, as electroactive materials, are useful sensors for this drug determination in its pharmaceutical formulation, Afrin nasal drops (0.05%). These sensors have advantages of high selectivity, low cost and fast response and wide applicable range.