Introduction
Periodontal disease is a biofilm-induced chronic inflammatory disorder.1-3 Its primary cause is the bacterial biofilm that triggers an inflammatory response, causing irreversible damage to periodontal tissues and consequent tooth loss if untreated.4,5
Thus, one of the aims of periodontal treatment is to reduce the number of pathogenic microorganisms in contact with periodontal tissues,2 including orange and red complex pathogens such as Porphyromonas gingivalis, Prevotella intermedia, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans.3
Non-surgical periodontal treatment is based on mechanical debridement,6 which is combined with subgingival irrigation to eliminate bacteria and improve its outcome.4,7,8 Periodontology is particularly concerned with using natural compounds, and propolis extract is an example found in solutions for subgingival irrigation. In-vitro studies have shown the susceptibility of periodontopathogenic microorganisms to solutions prepared from propolis extract.8
Propolis is the generic name for a type of glue, a complex resinous material that bees collect from the buds and exudates of plants to construct and repair honeycombs.4 Several biological activities have been attributed to propolis, including antifungal, antibiotic, antiprotozoal, antiviral, antitumor, and anti-inflammatory properties.5 The main chemical classes in propolis are flavonoids and phenolic and aromatic compounds, while volatile oils, terpenes, and bee wax do not significantly contribute to its properties or chemical effects.4,5,9-11
Despite the increasing use of propolis worldwide, few studies have evaluated the inhibitory activity of propolis against important dental pathogenic anaerobic bacteria, such as periodontopathogenic bacteria.9-11 The objective of this systematic review was to gather studies that used propolis extract to control antimicrobial activity and evaluate its in-vitro activity against major periodontopathogens.
Material and Methods
This report complies with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses.12 The protocol was not registered, given the nature of the investigated population.
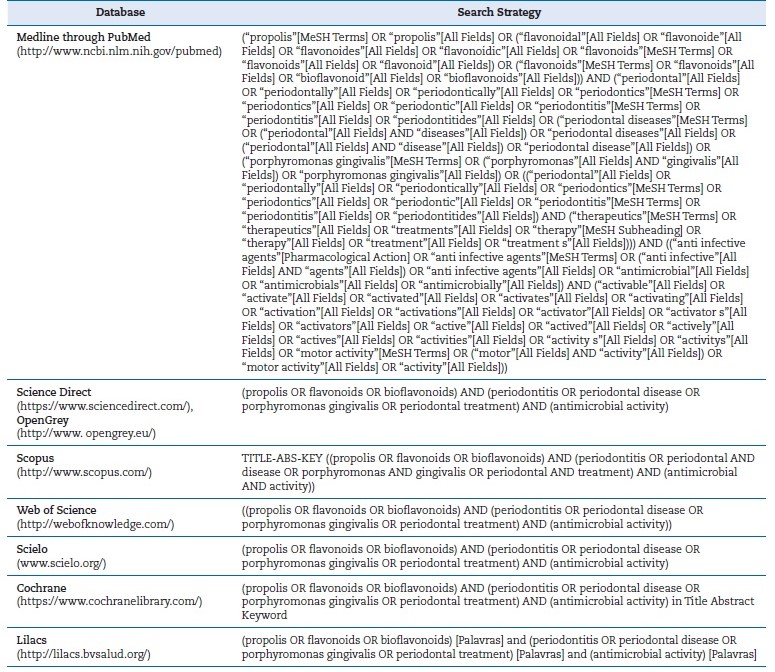
A search was performed on the PubMed/MEDLINE, Scielo, Lilacs, Science Direct, Cochrane, SCOPUS, and Web of Science databases, complemented by a manual search. We also searched the gray literature in Google Scholar and OpenGrey using appropriate strategies; only the first 100 articles of the Google Scholar search were analyzed. We searched for published studies evaluating the antimicrobial activity of própolis extract against at least one type of periodontopathogenic bacteria, using other substances already known as a comparison.
There were no language and time restrictions. The search was updated in January 2023. The search strategies are described in Table 1.
The eligibility criteria were as follows: in-vitro studies evaluating the action of propolis against Porphyromonas gingivalis; studies evaluating the antimicrobial activity of propolis using the minimum inhibitory concentration (MIC); studies whose full text was available. The exclusion criteria were in-vivo studies, case reports, case series, literature reviews, and systematic reviews. There was no language restriction for the search and inclusion of articles.
Two researchers (J. G. A. M. and D. M. S.) independently preselected the studies using the eligibility criteria to analyze the titles and abstracts. We first performed a calibration exercise with 20% of the retrieved studies, which confirmed the reviewers’ ability to analyze the remaining titles and abstracts to preselect articles. Then, they read the full text of the studies preselected, and those that met the eligibility criteria were included in the review. In case of disagreement between the two researchers, a third reviewer (S. C. S.) reevaluated the articles for possible inclusion in the study.
The following data were extracted from the included studies: year of publication, author(s), origin of the propolis samples, method of propolis extraction, oral bacterial strains used, and evaluation of antimicrobial activity based on MIC.
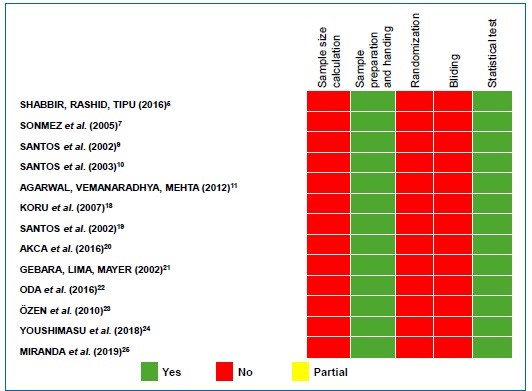
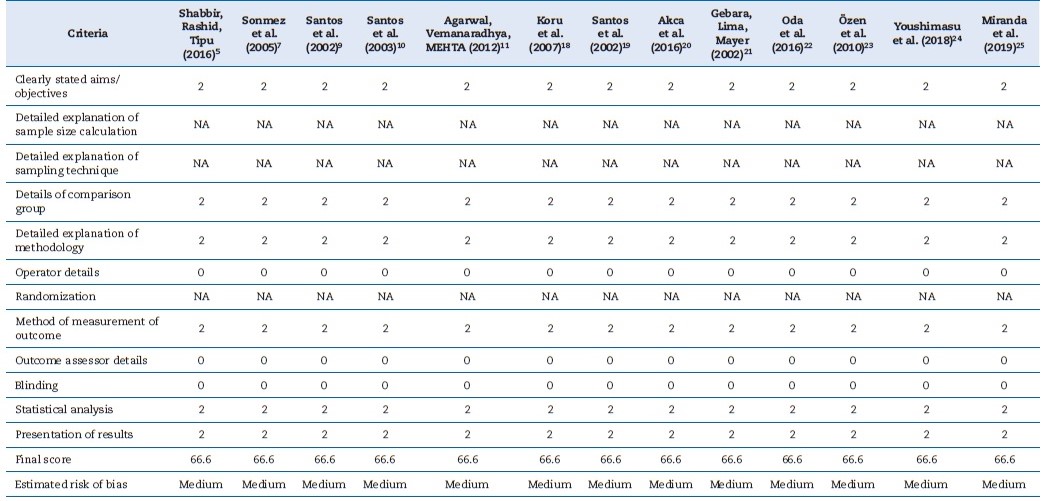
The quality of evidence on the included publications was assessed using the Database of Abstract of Reviews of Effects (DARE) tool13 and Checklist for Reporting In-vitro Studies (CRIS) guidelines.14 Five questions were considered: (i) Was the sample size calculation included?; (ii) Were sample preparation and handling adequate?; (iii) Were the samples randomized?; (iv) Was there blinding?; (v) Was a correct statistical test used? The interpretation for scoring “yes,” “partially,” and “no” is described in Figure 1. Two independent researchers (J. G. A. M. and D. M. S.) performed the quality assessment process and tried to resolve any dispute via discussion. If necessary, a third reviewer (S. C. S.) was consulted to reach a consensus.
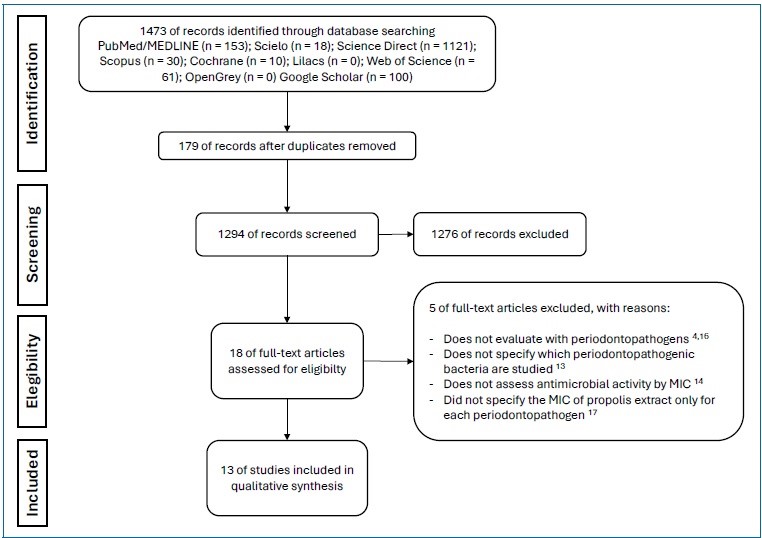
Figure 2 Assessment of the quality of evidence on the included publications using the Database of Abstract of Reviews of Effects (DARE) tool and the Checklist for Reporting In-vitro Studies (CRIS) guidelines
The risk of bias in the studies included was appraised using the QUIN method for in-vitro studies.15 Two reviewers (D. M. S. and J. G. A. M.) evaluated the QUIN tool’s 12 criteria. Each criterion was then assigned a score: 2 for “adequately specified,” 1 for “inadequately specified,” and 0 for “not specified.”
Criteria recognized as not applicable were not included in the final score. The studies’ risk of bias was considered low when the final score was above 70%, medium between 50% and 70%, and high below 50%.
Results
The database search resulted in the retrieval of 1473 articles. After analysis of the titles and abstracts, 179 duplicated articles were excluded, and 18 articles were selected for full-text screening, of which five were then excluded.4,14-17 Thus, this systematic review comprised 13 articles (Figure 2).5,7,9-11,18-25 The studies evaluated the antimicrobial activity of puré propolis against Porphyromonas gingivalis and other periodontopathogenic bacteria (Prevotella intermedia, Prevotella nigrescens, Fusobacterium nucleatum, and Aggregatibacter actinomycetemcomitans). They analyzed propolis collected in different regions of Brazil and the world, as well as the main oral bacterial strains. Table 2 summarizes the propolis samples’ region of origin, periodontal bacterial strains, and positive and negative controls used in the microbiological studies.
Table 2 Propolis samples used in the studies, techniques used for propolis extraction, bacterial culture, minimum inhibitory concentration (MIC) of propolis extract, and the main results reported in the studies evaluated.
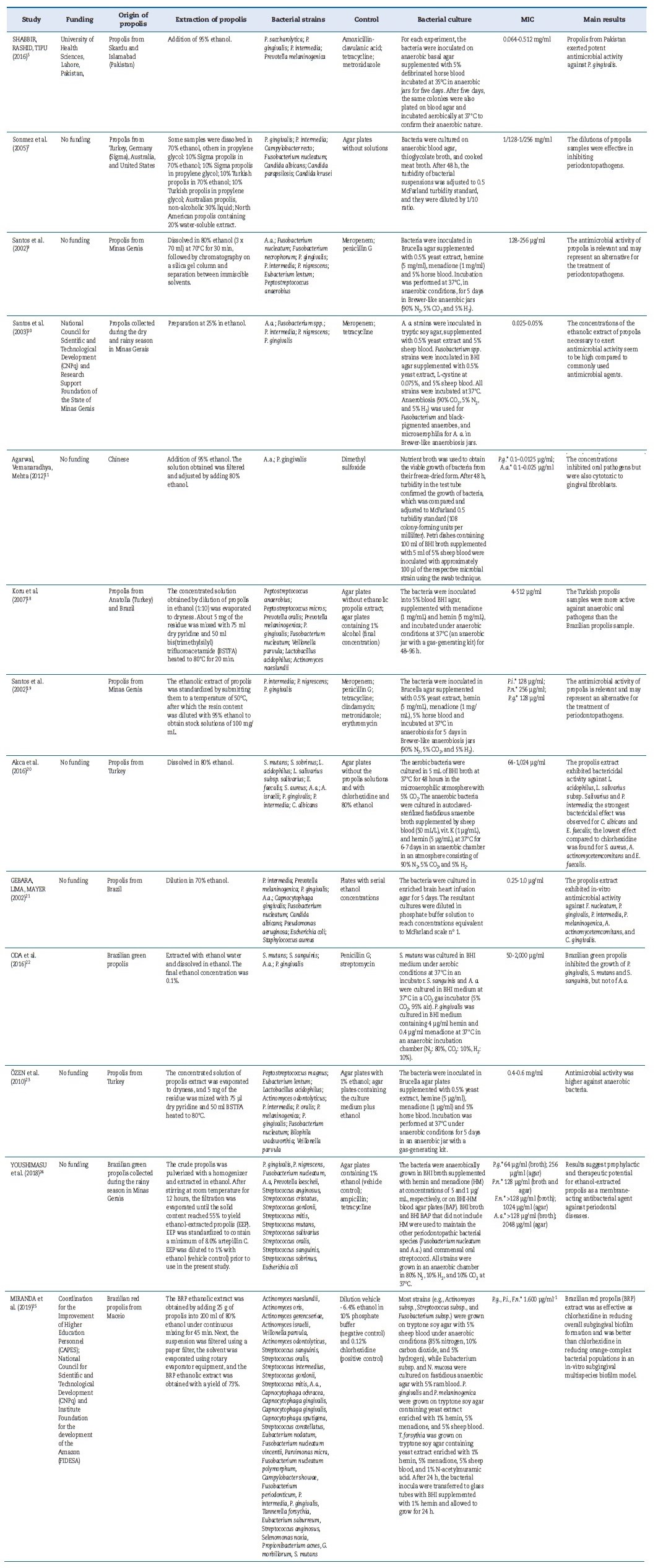
*P.g.: Porphyromonas gingivalis; A.a.: Aggregatibacter actinomycetemcomitans; P.i.: Prevotella intermedia; P.n.: Prevotella nigrescens; F.n.: Fusobacterium nucleatum. BHI: brain heart infusion
As indicated in Table 2, the crude propolis samples were diluted in ethanol to different concentrations, and the extracts were obtained using different techniques. The MIC was the lowest extract concentration to inhibit the visible growth of the test organism. The agar diffusion test was used in all studies to describe the MIC of antimicrobial activity.
All 13 articles included evaluated the action of própolis extract against P. gingivalis and other periodontopathogenic bacteria, including P. Intermedia,5,7,19,21,23 F. Nucleatum,7,10,18,21 A. Actinomycetemcomitans,10,11,20,22,25 and P. nigrescens.19,24 One of the articles also evaluated the action of propolis extract against the growth of C. Albicans.2
Five of the articles included used clinical use antibiotics, such as ampicillin,24 amoxicillin with clavulanic acid,5 clindamycin,19 tetracycline,5,10,19,24 meropenem,9,10,19 penicillin,9,19 metronidazole,5,19 and erythromycin,19 as comparison groups regarding propolis extract against the aforementioned microorganisms.
Articles by Shabbir et al.5 and Santos et al.19 reported the tested bacteria showed resistance to tetracycline5,19 and clindamycin,19 but were susceptible to propolis extract. In contrast, Santos et al.10 observed that all bacteria analyzed were susceptible to the antibiotics used (tetracycline and meropenem) and propolis extract.
Chlorhexidine, at 0.12%25 and 0.2% concentrations,20 was also used as a comparison. Miranda et al.25 found that própolis extract significantly reduced orange complex species, while chlorhexidine was less effective. The same authors reporte that both propolis extract and chlorhexidine significantly reduced the proportions of red complex specimens associated with periodontal disease. Akca et al.20 suggested that própolis extract would be more effective against P. intermedia than chlorhexidine.
In the quality of evidence assessment, no study indicated whether the sample calculation was performed or whether there was a randomization or blinding process for the MIC analysis. However, all studies explained how sample
preparation and handling were performed and whether a correct statistical test was used (Figure 2). Table 3 presents, in detail, the results of the risk of bias assessment of the studies included in this systematic review. All articles included presented a moderate risk of bias.
Discussion
The present systematic review evaluated studies that used propolis to inhibit the microbial activity of oral bacteria. Several antimicrobial agents are used as coadjuvants to mechanical therapy in periodontal disease. The choice of propolis was based on the increasing attention given to natural products.8
Among the coadjuvant therapies tested, propolis has shown antimicrobial properties, which justifies the investigation of this phytotherapeutic agent against periodontopathogenic bacteria. In Santos et al. study,9 P. anaerobius, P. gingivalis, and P. intermedia were the bacteria most susceptible to propolis, with MICs ranging from 128 to 256 μg/ml. Meropenem and penicillin G were used as positive controls, and their MIC ranged from 0.03 to 8 and 4 μg/ml, respectively. Compared to these values, the MICs of the ethanolic extract of propolis and its fractions do not appear to be significant. However, the antibacterial activity of propolis is highly significant since many of the bactéria tested are resistant to clinically used antibiotics.9
The systemic administration of antimicrobial agents can lead to multidrug-resistant microorganisms, transfer of resistance determinants, and side effects. Several studies have demonstrated the resistance of bacterial strains to tetracycline. In turn, clinical isolates resistant to tetracycline were found to be susceptible to propolis extract. One factor that may explain the lack of development of bacterial resistance to propolis is the wide variety of active agents in its composition associated with the need for synergism among these compounds.5,9,10,18,19,26 These essential characteristics permit própolis to act against diverse oral pathologies, reducing the prescription of antimicrobials and consequent bacterial resistance.
Brazilian green propolis with some isolated compounds, such as artepillin C, baccharin, and ursolic acid, causes a depolarization of the P. gingivalis membrane, making it more permeable.23 Yoshimasu et al.24 concluded that the combined therapy of Brazilian green propolis ethanolic extract with low doses of antibiotics would increase antibiotics’ effectiveness, but such synergistic activity should be further studied. The use of the Brazilian green propolis ethanolic extract with the main antibiotics in addition to periodontal treatment is, therefore, an interesting practice to reduce the adverse effects caused by antibiotics and their time of administration.
Ethanol extraction is the most popular method for the production of propolis extracts.27 Park et al.28 concluded that the 80% ethanol extract resulted in the largest quantity of flavonoids and that most flavonoids identified were extracted with 60 to 80%. Furthermore, the inhibition of microbial growth was considerably higher at this concentration. Based on these studies, it would be interesting to standardize the concentration of ethanol for propolis extraction in order to obtain adequate antimicrobial activity for propolis collected in any region. Propolis extract can be obtained using different techniques.
The traditional method is maceration, which is slow, requiring 2 to 10 days.29,30 Extraction by ultrasound is faster and more efficient.31 It is important to establish the best technique for extracting samples collected in different regions to obtain the highest antimicrobial activity.
The subgingival microbiota is dominated by Gram-negative rod-shaped bacteria.32 Koru et al.18 observed that the MICs of anaerobic Gram-positive bacteria were lower than those of anaerobic Gram-negative bacteria; thus, the latter were more sensitive to the ethanolic extract of propolis. In contrast, in the studies of Akca et al.20 and Grange and Davey,33 the results of biofilm analysis did not confirm that finding, as própolis was more effective against Gram-positive bacteria. Koru et al.18 also found that all anaerobic bacterial strains were susceptible to the ethanolic extract of propolis, regardless of its region of origin.
Oda et al.22 demonstrated a weak inhibitory effect of Brazilian green propolis on A. actinomycetemcomitans. Bacterial growth was observed up to the highest concentration of 2,000 μg/ml, the dose limit that does not cause cytotoxic effects on gingival fibroblasts. Sonmez et al.7 found that Australian própolis (non-alcoholic, 30%) and North American propolis (alcoholic, 20%) effectively inhibited antimicrobial activity, but the concentrations tested were cytotoxic to gingival fibroblasts. Agarwal et al.11 evaluated Chinese propolis, and the concentrations that inhibited oral pathogens, including P. gingivalis, were also cytotoxic to gingival fibroblasts.
The studies by Miranda et al.25 and Figueiredo et al.26 show that the ethanolic extract of Brazilian red propolis has a significant effect on multispecies biofilms of pathogens associated with periodontal disease and can be a great ally when associated with non-surgical periodontal treatment.
The bacteria used in experimental studies can be derived from clinical isolates or reference strains. Most papers included in this study used reference strains.7,9,11,18,20-24 None of the included articles assessed only clinical isolate strains, but the studies by Santos et al.9, Santos et al.,10 and Shabbir et al.5 used part of bacterial strains from clinical isolates and part of reference strains.
It is understood that periodontal diseases result from a “dysbiotic” biofilm and not directly from the effect of specific pathogenic bacteria on the host. However, some key pathogens, such as Porphyromonas gingivalis, play an important role in the imbalance with the host, increasing the pathogenicity of the bacteria in the biofilm and inducing a disease state.34
This understanding of the polymicrobial dependence of a dysbiotic biofilm for the emergence of periodontal diseases indicates the need to carry out more experimental studies that use a microbial environment similar to those found in individuals with periodontal disease. Using isolated strains may not configure an evaluation of the periodontal pathological condition close to the real one.
One of this study’s limitations is that the quality of evidence of the studies included in this systematic review was not assessed using GRADE because in-vitro studies do not generate evidence with immediate clinical application. Besides, several points analyzed by the GRADE tool cannot be applied in a methodology of experimental studies in vitro.
Conclusions
In conclusion, the results show that propolis extract exhibits effective antimicrobial activity against the main oral bactéria and might be a feasible therapeutic option for the modulation of periodontitis, in addition to reducing the use of antibiotic therapy for oral pathogens and consequently the risk of bacterial resistance. Further studies are important to better understand the mechanisms of propolis’ anti-inflammatory and antimicrobial actions and analyze the cytotoxic effects of diferente concentrations on gingival fibroblasts.