Introduction
According to the European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, core needle biopsy (CNB) of the breast can be classified into 5 categories based on lesion type and malignancy risk1, with the normal (B1), benign (B2), or malignant (B5) categories accounting for approximately 85-90% of diagnoses2,3. While a B4 diagnosis means suspicious for malignancy, with studies reporting a positive predictive value (PPV) for carcinoma as high as 88%4, the B3 category, which comprises around 5-10% of CNB diagnoses in most series2-5, has a lower PPV for malignancy depending on lesion type, but overall ranging from 10-35%4-15, which highlights its uncertain potential and complex management.
Most B3 lesions are ultimately benign but even when malignancy develops at these sites, it usually occurs over an extended period with a low histological grade and hormone receptor positivity16. Despite this, underestimation rates for CNB are not neglectable, making it crucial to identify features that help narrow down malignancy risks and tailor treatment strategies.
Pathologists classify B3 lesions into several histotypes, six of which are noted as the most relevant17. These include atypical ductal hyperplasia (ADH); flat epi-thelial atypia (FEA); classical lobular neoplasia (LN), which can be divided into atypical lobular hyperplasia (ALH) and lobular carcinoma in situ (LCIS) based on the extent within the terminal ducto-lobular unit (<50% and ≥50%, respectively); papillary lesions (PL); radial scars (RS) (≤1.0 cm)/complex sclerosing lesions (CSL) (>1.0 cm); benign and borderline phyllodes tumors (PT) (12.
Vacuum-assisted biopsy (VAB) devices offer the advantage of obtaining large tissue samples (approximately four grams), comparable to a surgical biopsy, thereby increasing representativeness and sometimes completely excising lesions. They can be used to re-evaluate a CNB that is not representative of the lesion on imaging or to assess B3 lesions for upgrades, with a lower risk of discordance with the final surgical diagnosis. The trend to make the treatment for B3 lesions minimally invasive began by using these devices to undertake a vacuum-assisted excision (VAE) of non-atypical RS and PL, offering a minimally invasive alternative to surgery that benefits patients and reduces healthcare costs18.
The recent “Third International Consensus Conference on lesions of uncertain malignant potential in the breast” reviewed current literature and recommended that B3 lesion management with surgery or clinical and radiological follow-up be individually discussed in multidisciplinary meetings, considering imaging findings, histopathological features at CNB or VAB, patient risk factors for an upgrade, and life expectancy17.
VAE is now preferred over open excision (OE) for certain B3 lesions diagnosed via CNB, such as FEA, PL without atypia, RS/CSL, and LN. However, OE remains the preferred approach for ADH due to its higher risk of malignancy upgrade, and for PT, even if an upgrade is uncommon after CNB or VAB, since the definitive histological diagnosis can be best rendered on OE specimens where excision completeness may be assessed17.
Our study aimed to clarify the clinical significance of B3 lesions and contribute to determining the best approach for their treatment and follow-up. To this end, we investigated overall and subtype- specific malignancy risks and sought to identify variables that could predict the presence of malignancy, while comparing results between different excision methods and follow-up-only groups.
Methods
This retrospective study reviewed a single-center series of patients being followed in the context of a histological diagnosis of a B3 lesion, between November 2018 and November 2023. Only patients without a syn-chronous higher-grade lesion diagnosis were included. This study was approved by the local ethics committee [2023.248(210-DEFI/200-CE)].
The Kolmogorov-Smirnov and Shapiro-Wilks tests were used to evaluate normal distribution. A descriptive analysis of the patient’s clinical, radiological, and pathological characteristics was performed, using numbers and percentages for categorical variables and medians with interquartile ranges for continuous variables as normal distribution was not found in most. Data retrieved from hospital records includes: family history, which was categorized into a postulated higher-risk subset (history of breast or ovarian cancer in at least three direct family members, at least one below the age of forty or at least one from the male sex) (19, and a low to intermediate-risk subset (any other kind of family history); radiological suspicion score according to the Breast Imaging Reporting and Data System (BIRADS); lesion foci, where multifocal lesions were defined as two or more foci within the same breast quadrant, and multicentric lesions as those in different quadrants20.
For patients who underwent excisional management, the pathology reports of final specimens were analysed to correlate with the primary results. An upgrade was reported in the presence of invasive or in situ carcinoma on final histology. Follow-up imaging and histopathological exam reports were reviewed when available and lesion recurrence was classified as concordant based on either of the exams, while only the latter confirmed upgrades or different B3 lesions. We defined recurrence as both the persistence of abnormal radiological findings in subsequent imaging reports (incomplete excision) and as the presentation of de novo suspicious radiological findings after complete excision had been documented.
For continuous variables, the Independent Samples T-test, One-way ANOVA, Mann-Whitney, and Kruskal-Wallis tests were used according to whether normal distribution was verified and the number of variables being compared. For categorical variables, Pearson’s Chi-square test, the Monte Carlo exact test, and Fi-sher’s exact test were used, as appropriate, to assess the association between patient characteristics, diagnostic or treatment strategies, and malignant upgrade. Statistical analyses were performed using IBM SPSS 29.0 software for Windows (IBM SPSS, Armonk, NY, USA). The considered statistical significance was p<0.05.
Results
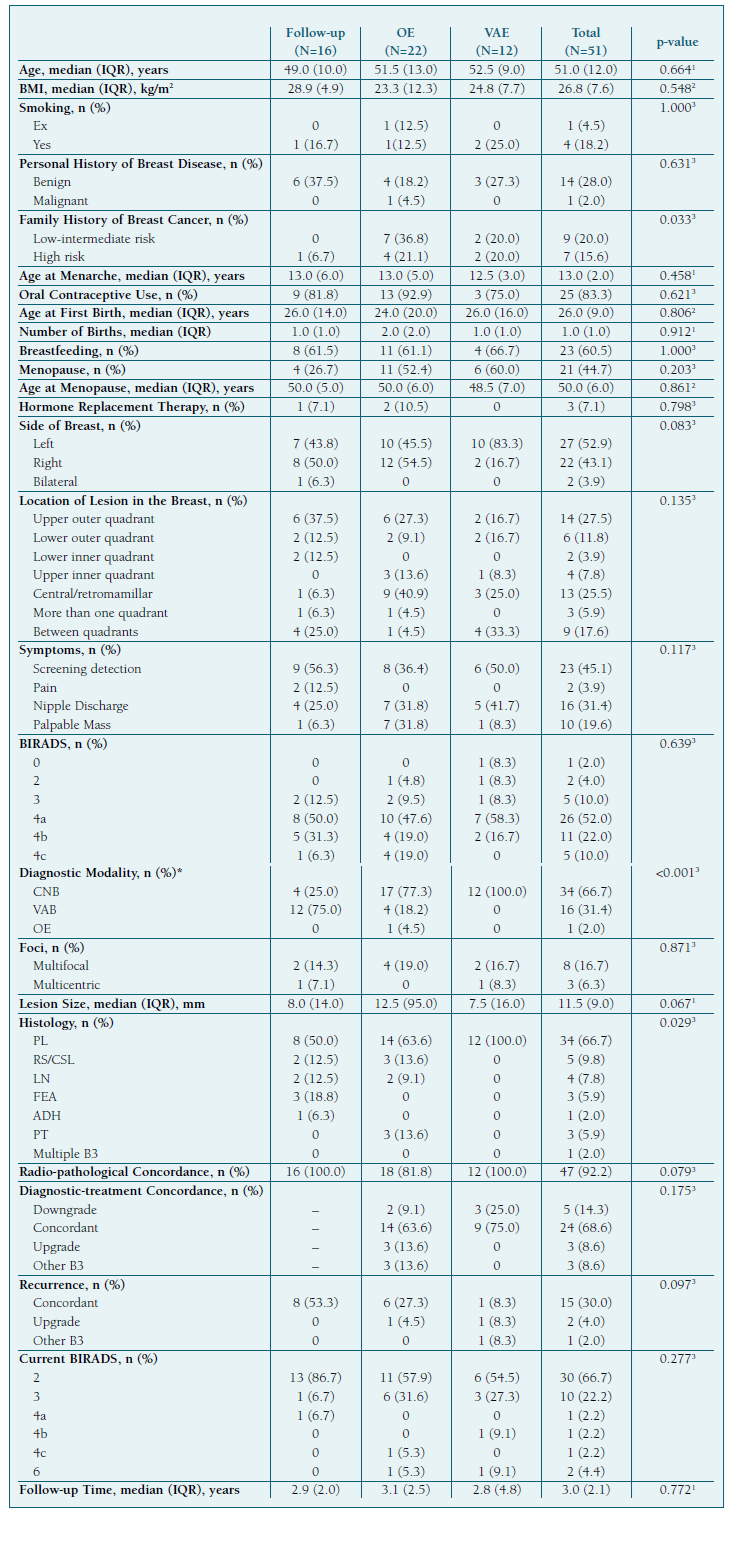
51 patients diagnosed with one or more B3 lesions and no concomitant malignancy were included in this study. All individuals were female and alive at the time of data retrieval. We categorized patients by clinical management: 16 (31.4%) opted for active surveillance, while the remainder underwent lesion excision, either through OE in 22 patients (43.1%) or VAE in 12 (23.5%). Additionally, 1 patient underwent bilateral skin-sparing mastectomy (BSSM) in the context of multicentric PL associated with LCIS. There was no surgical upgrade of the lesions or evidence of malignancy on follow-up. This patient was omitted from the statistical tests but included in the total group descriptives.
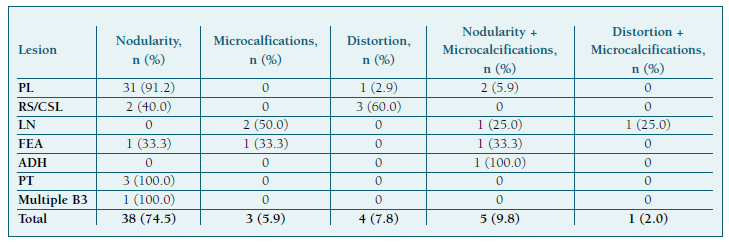
The median patient age was 51 years (IQR=12.0), 21 (44.7%) were postmenopausal, and 28 (54.9%), presented with breast symptoms. Most lesions were detected in the upper outer quadrant (14 cases; 27.5%), closely followed by the central/retromamillary region (13 cases; 25.5%), where all lesions were subcategorized as PL (Table I). Most lesions could be described as nodularity (38 cases; 74.5%), while microcalcifications were found in 9 patients (17.7%) (Table II). Of patients with a BIRADS equal or inferior to 3, 7 (87.5%) had PL, and 1 (12.5%) had FEA. Most PL cases presented with a 4a BIRADS (22 cases; 66.7%), LN with a 4b BIRADS (3 cases; 75.0%), and RS/CSL with a 4c BIRADS (3 cases; 60.0%).
Most B3 lesions were diagnosed through CNB (34 cases; 66.7%), 16 (31.4%) through VAB, and 1 (2.0%) through OE, which was both diagnostic and therapeutic in the context of a lesion suspected of being a PT. Among B3 lesion subtypes, PL were the most frequent (34 cases; 66.7%), and 1 patient (2.0%) had multiple B3 subtypes at diagnosis (PL and LN) (Table I).
Lesion treatment outcomes included 5 downgrades to benign or normal breast tissue (14.3%), 24 concordant results (68.6%), 3 upgrades (8.6%), and 3 different B3 lesions detected (8.6%). Patients had a median follow-up time of 3 years (IQR=2.1) and during this period 15 (30.0%) had evidence of lesion persistence or recurrence, 2 (4.0%) had an upgrade, and 1 (2.0%) had a different B3 lesion (CSL) diagnosed after prior diagnosis and treatment of a PL (Table I).
We found statistical significance between the chosen management modality and the patient’s family risk for breast cancer (p=0.033), with OE reporting the highest sum of patients at increased risk, 11 (57.9%), and only 1 patient (6.7%) on the follow-up-only group. It also seems to be related to the used diagnostic tool (p<0.001) when excluding the one patient with a diagnostic and therapeutic OE from the analysis. Most patients in the follow-up-only group had a previous VAB (12 cases; 75.0%), those who underwent OE were mostly diagnosed through CNB (17 cases; 77.3%), and all 12 (100.0%) who underwent VAE were diagnosed through CNB. Finally, we found a correlation with the histological diagnosis (p=0.029). All 12 patients who underwent VAE had a prior diagnosis of PL. From ano-ther perspective, all 3 patients diagnosed with PT underwent OE, and all 3 diagnosed with FEA were kept on follow-up (Table I).
Despite the statistical analysis not indicating other significant differences between management cohorts, it is relevant to mention that radio-pathological concordance was verified in all follow-up and VAE patients, compared to 18 (81.8%) treated with OE, meaning that this was the chosen modality in case of discordance. As for diagnostic-treatment concordance, no patients who underwent VAE had an upgrade or different B3 lesions on final histology but these outcomes were each found in 3 (13.6%) patients who had an OE. We found lesion recurrence in each management subgroup, all of which were classified as concordant in the follow-up-only group (8 cases; 53.3%), most of which were concordant in the OE group (6 cases; 27.3%), and were evenly distributed in the VAE group with 1 patient (8.3%) either having a concordant result, an upgrade or a different B3 lesion. The 1 patient with an upgrade was the only VAE patient who did not avoid surgery. Overall, recurrence was found in 7 cases (31.8%) for the OE cohort and 3 (24.9%) for the VAE cohort.
When grouping patients by malignancy presence in the final specimen, no significant differences were found. However, adding the 2 follow-up malignancy cases to create a “total upgrade” group revealed statistically significant associations with histological type on the diagnostic biopsy (p=0.020, Monte Carlo exact test), LN being particularly more associated with upgrades (p=0.017, Fisher’s exact test), number of lesion foci (p=0.023, Monte Carlo exact test), and a BIRADS category of at least 4b at diagnosis (p=0.019, Fisher’s exact test).
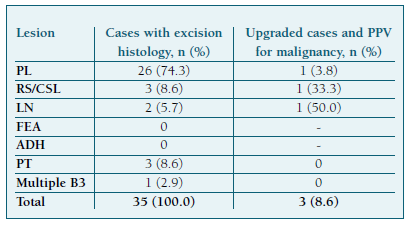
Lesion-specific PPVs for an upgrade are presented in Table III. LN and RS/CSL exhibited high malignancy risks, with a PPV of 50% and 33.3% respectively, while PL had a low PPV of 3.8%. PPVs for ADH and FEA could not be determined, as none were excised.
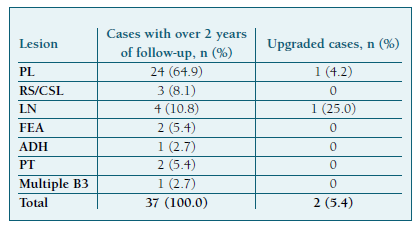
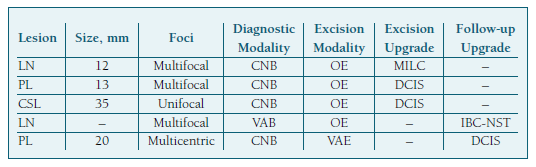
Concerning future breast cancer development risk after a B3 diagnosis, 2 of 37 (5.4%) women with at least a 2-year follow-up developed cancer in either breast. Despite the small sample size, we found a risk of 4.2% for patients with PL and 25.0% for LN (Table IV). These patients respectively developed DCIS and invasive breast carcinoma of no special type (IBC-NST) and were both diagnosed and excised through different methods (Table V).
Discussion
The malignization rate of 8.6% in our study is slightly below the reported range of 10-35%. However, it aligns with the upgrade rate reported in a study focused on lesions excised through VAE16. Several factors could explain this lower rate, including the distribution of B3 histotypes: while in most studies ADH, the B3 lesion associated with the highest upgrade rates, comprises the most common lesion subgroup9,11,13,14, in our study, PL were much more prevalent. One study reported a lower incidence of malignancy in surgical excision following the initial VAB diagnosis of ADH than after the CNB diagnosis21. The fact that 31.4% of patients were diagnosed through VAB and less than half were managed surgically (45.1%), meaning that most did not meet severity criteria, may be other contributing factors.
The differentiation of B3 subtypes can impact patient outcomes. In our study, of the 2 excised LN, 1 (50.0%) had an upgrade, indicating the highest upgrade risk among B3 lesions. Variability in upgrade rates across studies, ranging from 4-67%17, may be due to partially small case numbers or separate evaluation of LN or together with simultaneously present high-risk lesions like ADH22. A possible reason for such a high upgrade rate in our study is a preselection of cases in the multidisciplinary conference, where surgical excision was mostly recommended for cases with radio-pathological discrepancies. Lower malignancy rates of 3-8% refer to larger case numbers22 or radio-pathological concordance23.
The 1 identified ADH case (2.0%) in our study deviated significantly from larger multicenter studies, where it accounted for up to 40% of all B3 lesions24. Given this small sample, it was impossible to determine the PPV for malignancy. Current literature mentions an upgrade rate of 7.3-57%, recommending excision after biopsy, especially since the differential diagnosis with DCIS is based solely on lesion size17,25. Nevertheless, other options such as imaging follow-up after VAB, when the calcifications in clinical imaging have been completely removed, are being increasingly considered17. This was the case for our patient, with no recurrence detected during follow-up.
PL were the most frequent lesions in our study (66.7%), with an upgrade rate of 3.8%. Pure intraductal PL upgrade rates range from 1-9%, whereas lesions with atypia can have an upgrade rate of up to 38%17. Consequently, radiological surveillance seems appropriate for smaller or completely excised benign PL and VAE poses an alternative to OE. Even though the presence of atypia was not recorded for PL in this study, most were likely pure PL. Radio-pathological discordance, symptom presence, age ≥60 years old, size ≥10 mm, peripheral location, calcifications, and presence of ≥4 PL foci constitute factors associated with malignancy and should be considered when deciding on elective surgery26. Our study found that of 24 PL that were followed for at least 2 years after diagnosis, 4.2% were upgraded (DCIS), while a recent study that included 139 non-excised PL found an upgrade rate of 1.4% within 2 years of diagnosis27.
RS/CSL had a 33.3% upgrade rate in our study. Recent research on a large patient cohort found a 9% upgrade rate in RS/CSL without atypia, compared to 33% in those with atypia28. Further studies revealed low upgrade rates (0.9-1.6%) associated with increased VAE use after CNB diagnosis of RS without atypia17. In contrast, our study did not use VAE and only 2 VABs were performed in 5 diagnosed lesions, likely due to radio-pathological discordances. Some researchers have identified large radiographic size as a risk factor for cancer upstaging of RS/CSL29, with an average size of 1.4 cm30. In our upgraded case, the lesion had a significant 3.5 cm diameter, supporting the idea that CSL carries a higher upgrade risk than RS.
FEA was found in only 3 (5.9%) patients, of which no final histology specimens were obtained, preventing the determination of PPV. The literature reports low malignancy rates under 10% and there is consensus that surgical excision may be unnecessary if a larger gauge biopsy is utilized17. This has been the approach at our center, where these lesions were kept on follow-up after mostly being diagnosed through VAB (66.7%).
All PT in our study group underwent surgical excision. None were upgraded in the final histology, leading to an estimated 0% PPV for malignancy. In our study, 2 PT were benign and 1 was classified as borderline. Regardless of the PT subtype, surgical extirpation or even radical mastectomy should be performed due to the difficulty in differentiating PT from benign fibroadenoma in incomplete samples31.
While most of our patients seem to have been selected for follow-up-only, it is important to clarify that most of their lesions were diagnosed through VAB and either obtained complete excision on follow-up imaging or asymptomatic lesions with no suspicion criteria remained present. The 4 patients who had a CNB with no other medical interventions had either benign PL not identifiable on ultrasound for VAE, a clinically stable mass with FEA, a small stromal distortion diagnosed as RS without atypia or because that was the patient’s preference.
Another important finding of our study was the 5.4% malignancy development rate during at least a 2-year follow-up. No malignancy was found before then. The 10-year absolute risk of breast cancer is 2.85% in 50-year-old females in the general population32. Some recent studies evaluating age- specific 10-year absolute risk, with the goal of risk-stratifying breast cancer screening, indicated a threshold of 6% to define “high-risk” women33,34. Our follow-up malignancy risk below 6% does not provide evidence that everyone with a B3 diagnosis should be defined as “high-risk”. Instead, surveillance should be tailored based on lesion subtype and individual risk factors. In the literature, the three histotypes with a higher risk of future cancer were ADH (13.6%), atypical PL (9.7%), and LN (8.8%) and there is consensus that these should not be discharged from clinical and radiological follow-up11. Our study had a limited population of these subtypes, but the 25% malignization rate for LN suggests it should be considered high-risk. High-risk women might benefit from annual breast magnetic resonance imaging screening for at least 5 years11.
Imaging findings classified as BIRADS 4b or 4c7, lesion sizes over 6 mm35 or 10 mm36, and older postmenopausal woman15 were significant upgrade predictors. When we grouped all patients who had an upgrade on the final specimen or during follow-up, we found that a BIRADS category at diagnosis of at least 4b, multifocality, and a LN histological subtype were significantly associated with the malignancy outcome, which aligns with previously mentioned risk factors.
VAE provides excellent cosmetic results, high patient satisfaction, avoids risks of general anesthesia, and is low-cost16. Studies indicate that VAE of B3 lesions reduces the number of open surgical procedures37. In our study, 12 women underwent VAE, of which 91.7% avoided further surgery. This higher rate of surgery avoidance may be due to case mix and a cautious approach to recommending VAE after multidisciplinary team discussion.
Our study has a few limitations: several patients had incomplete data; risk factors could only be evaluated concerning all B3 lesions due to the small numbers of individual subtypes; PL with or without atypia were not differentiated; short median follow-up time of 3.0 years. Further research is needed to determine the appropriate length of mammographic follow-up, as new studies challenge previous conservative approaches38.
Acknowledgments
The authors are grateful for the exceptional support of Professor Carolina Lemos who supervised the statistical review.