Introduction
Congenital vascular malformations present a broad phenotypic spectrum, ranging from skin changes with exclusive capillary involvement to large arteriovenous communications potentially limb and life-threatening.1,2 Arteriovenous malformations (AVMs) are a rare subgroup, characterized by high-flow communication between the arterial and venous systems through an aberrant vascular nidus that has replaced the normal capillary bed.3 4,5,6 They typically present at a young age and with reported progression rates of up to 96%, especially during puberty.3,6,7 Uncertainties regarding the pathophysiology and natural history associated with the lesion’s high complexity often culminate in poor therapeutic planning8,9 and AVMs are historically associated with extensive resection surgeries with high morbidity and recurrence, regardless of the approach.9,10 The different classifications, although not perfect, aim not only the categorization of the different vascular malformations but also allow a better understanding of their pathophysiology (Modified Hamburg Classification11 - Table 1.), clinical course (Schobinger Classification12 - Table 2.) and angioarchitecture (Yakes Classification12 - Table 3.). Thus, they can support the clinical decision in the absence of other specific guidelines. The evolution of the endovascular technique marked a turning point in the treatment of this pathology. Initially intended as an adjunct to conventional surgery, this approach has been gaining ground as a first-line therapy. This study aims to evaluate the results of embolization of arteriovenous malformations as a first-line approach in infiltrative lesions, unresectable by conventional surgery.12
Table 1 Hamburg Classification
| Congenital Vascular Malformations - Types |
|---|
| Arterial defects |
| Venous defects |
| Arteriovenous shunting defects |
| Combined vascular defects - hemolymphatic defects |
| Capillary defects |
| Congenital Vascular Malformations - Embryologic subtypes |
| Extratroncular forms |
| Infiltrating, diffuse |
| Limited, localized |
| Troncular forms |
| Stenosis or obstruction |
| Hypoplasia, aplasia, hyperplasia |
| Membrane, congenital spur |
| Dilation |
| Localized (aneurysm) |
| Diffuse (ectasia) |
Modified from the original classification based on the consensus reached at the international workshop in Hamburg, Germany, 1988
Table 2 Schöbinger Staging of Arteriovenous Malformations
| I (quiescent) | Warm areas of pink-blue discoloration |
| II (expansion) | Mass associated with thrill and bruit |
| III (destruction) | Mass associated with pain, bleeding and ulceration |
| IV (decompensation) | High output cardiac decompensation |
Table 3 Yakes Arteriovenous Malformation Classification
| I | Direct AVF |
| IIa | Typical AVM nidus |
| IIb | AVM nidus with shunt into an aneurysmal vein |
| IIIa | Aneurysmal vein whereby the vein wall is the nidus with single outflow vein |
| IIIb | Aneurysmal vein whereby the vein wall is the nidus with multiple outflow veins |
| IV | Infiltrative form with constituted by numerous micro-fistulae |
AVF - arteriovenous fistulae; AVM - arteriovenous malformation
Methods
A retrospective analysis of the clinical records of patients with infiltrative arteriovenous malformations who underwent embolization at our tertiary institution between 2019 and 2021 was performed. Demographic, clinical, procedure-related and postoperative outcome data were extracted from medical records.
The Schöbinger Classification (Table 2) was applied to categorize the clinical stage. Angiographic findings were independently evaluated by 2 authors (Pinelo A. and Loureiro L.) and reported according to the Yakes classification (Table 3.). Differences were resolved through consensus. The decrease in the Schöbinger grade after treatment, the number of reinterventions and associated complications were the main outcomes.
Considering the small number of patients included in this series, only a descriptive statistical analysis was performed. Table 4 e 5.
Table 4 Demographic and clinical characteristics
| Case | Sex | Referral age (y) | Age at treatment (y) | Location | Follow-up time (months) | YAKES classification |
|---|---|---|---|---|---|---|
| 1 | M | 15 | 15 | Inguinal | 18 | IIa |
| 2 | F | 6 | 7 | Suprascapular | 9 | IIa |
| 3 | F | 5 | 8 | Thigh | 9 | IIa |
| 4 | F | 10 | 19 | Buttock | 9 | IIa |
| 5 | M | 20 | 22 | Buttock | 13 | IIa |
| 6 | F | 22 | 28 | Foot | 37 | IV |
| 7 | F | 27 | 40 | Foot | 34 | IV |
| 8 | F | 3 | 4 | Leg | 27 | IIb |
| 9 | M | 43 | 45 | Thigh | 14 | IIa |
Table 5 Procedure-related characteristics
| Case | Access | Embolization material | Nr. of interventions | Schöbinger (pre-embolization) | Schöbinger (post-embolization)höbinger(post-embolization) | Complications | Surgical Resection |
|---|---|---|---|---|---|---|---|
| 1 | TA | Polidocanol Onyx18® | 2 | III | II | Ø | no |
| 2 | TA | Polidocanol Microcoils | 1 | II | Ø | Ø | no |
| 3 | TA | Polidocanol Microcoils PVA 500-710 | 1 | II | Ø | skin necrosis | no |
| 4 | TA | PVA 500-710 | 1 | III | III | Ø | no |
| 5 | TA | Polidocanol Onyx18® | 2 | III | I | Ø | no |
| 6 | DNP TA TV | Polidocanol Microcoils Onyx34® PHIL® | 5 | III | III | Ø | no |
| 7 | TA TV DNP | Polidocanol Onyx® Coil | 4 | III | I | tissue necrosis | yes |
| 8 | TV | Polidocanol | 1 | II | I | Ø | yes |
| 9 | TA | Onyx18® | 1 | II | Ø | Ø | no |
DNP - direct nidus puncture; TA - transarterial; TV - transvenous.
In patients with type IIa AVM (n=6) the nidus was embolized via transarterial approach with polidocanol (n=2), polidocanol + microcoils (n=1), polidocanol + microcoils + PVA 500-710microns (n=1), PVA 500-710microns (n=1) and Onyx18® (n=1) (Figure 1). The two patients treated with polidocanol alone required a second embolization, also performed via transarterial with Onyx18® 8 and 9 months after the first procedure. A reduction in Schöbinger's stage was achieved in 5 (83.3%) of the 6 patients in this group, with complete clinical resolution in 3 (50%).
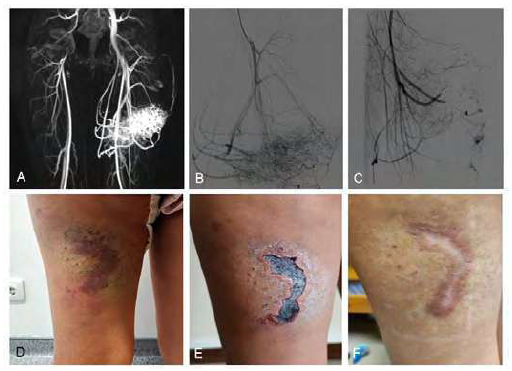
Figure 1 Yakes type IIa arteriovenous malformation (AVM) of the thigh. A) magnetic resonance angiography (MRA) showing infiltrative AVM with afferent branches from the deep femoral artery; B) pre-treatment angiography; C) final result after nidus embolization with polidocanol (3%) and PVA 500-710microns and afferent obliteration with Concerto® microcoils (2x40(x2); 2x60; 2x80; 3x40(x2); 3x80); D) pre-treatment macroscopic findings; E) post-intervention necrosis of the overlying skin; F) complete healing by the 3rd month after embolization
Type IIb AVM (n=1) was embolized with polidocanol foam via transvenous approach. A reduction of volume and Schöbinger's stage from II to I was achieved. Surgical excision of the residual lesion was performed 1.5 months after embolization, without recurrence at two-year follow-up.
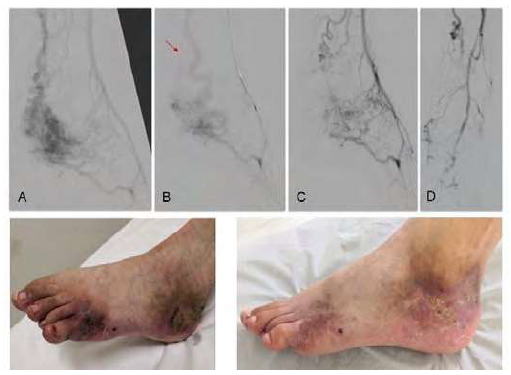
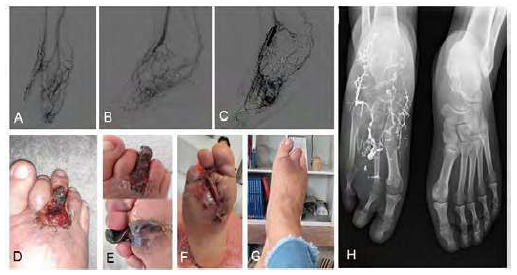
Type IV AVMs (n=2), both located in the foot, were the most complex lesions and had the highest number of interventions. In the first case, it was initially attempted polidocanol sclerosis through direct nidus puncture. Due to the absence of involution, the procedure was repeated four times with transarterial and transvenous embolization with polidocanol and PHIL®; an additional obliteration of the afference was carried out by placing a microcoil filled with Onyx34® in the posterior tibial artery and of the efference through anterior tibial vein sclerosis with polidocanol (Figure 2). It was achieved a clinical regression of the AVM with the favorable healing of ulcerated areas 30 days after the last procedure. In the second patient, four procedures were required with polidocanol and Onyx18® embolization via transarterial, transvenous and direct nidus puncture (Figure 3). There was involution of the lesion with delimitation of extremity necrosis zone requiring toe amputation. During a two-year follow-up after the last intervention, it persists a residual cutaneous malformation being treated with 940nm vascular laser therapy intermittent sessions.
A) pre-treatment angiography, inflow through branches from the posterior tibial, peroneal and pedal arteries; B) angiography after transarterial nidus embolization with polidocancol (3%) foam and posterior tibial artery obliteration with Concerto® 4x100 microcoil (arrow) and Onyx34®; nidus access was not compromised given the additional afference by peroneal and pedal arteries’ branches; C and D) angiographic result after transvenous sclerosis with polidocanol (3%) foam of the efferent anterior tibial vein (C) and transarterial nidus embolization nidus with PHIL® 30% via peroneal artery (D), performed during the same procedure; E) pre-treatment clinical findings with painful foot ulceration; F) healing ulcerations after last treatment.
A) pre-treatment angiography; inflow throw anterior and posterior tibial arteries branches; B) angiography after transarterial coil obliteration and Onyx18® embolization of plantar inflow branches, through posterior tibial artery and great saphenous vein sclerosis with polidocanol foam (3%); C) angiography after direct nidus Onyx18® injection followed by embolization of posterior tibial artery plantar branches and distal pedal artery obliteration, both with Onyx18®; D) clinical presentation with toe painful ulcer; E) toe necrosis after the last embolization treatment; F) atypical transmetatarsic toe amputation and residual AVM excision; G) Completely healed and functional stump with resolution of pain complaints; H) post-amputation X-Ray
Overall, the median number of interventions per patient was 1 (ranging from 1 to 5) and Yakes type IV appears to be associated with a higher rate of reintervention. Reduction of the Schöbinger stage was achieved in seven out of the nine patients (77.8%) patients, with clinical resolution in three (33.3%). We report a 11.8% (n=2) complication rate, both involving tissue necrosis. One patient with Yakes type IIa AVM treated with transarterial nidus embolization (Figure 1-C) complicated superficial necrosis of the overlying skin (Figure 1-E) which detached and completely healed (Figure 1-F). The other patient, presenting with a type IV Yakes AVM, developed complete toe necrosis (Figure 3-E) after transarterial and direct nidus embolization and pedal artery obliteration (Figure 3-C). We performed an atypical transmetatarsic 3rd and 4th toes amputation with excision of the residual nidus (Figure 3-F) which completely healed, preserving a functional foot. There were no other complications reported associated to the procedures.
Discussion
Arteriovenous malformations manifest at a young age with an unpredictable and potentially life-threatening course. There are no specific guidelines for treatment and, while some centers recommend intervention in stages III and IV of Schöbinger,13 others adopt an earlier approach due to the high risk of progression1. There is little consensus on the best approach and it is difficult to establish a comprehensive strategy given the pathology’s clinical variability and complex stratification. Conventional surgery with complete resection is still the most effective treatment in small and localized lesions1,2 but it becomes less suitable for extensive and infiltrative lesions.13 The evolution of endovascular techniques has allowed new therapeutic perspectives, both as a bridge to conventional surgery and also as a primary therapy for lesions that cannot be surgically resected. The results of AVM embolization described in the literature are divergent and difficult to interpret given the absence of a clear stratification and standardized criteria. Also, most studies include only AVMs of the head and neck, presumably due to the complexity of surgical resection in this location, turning the use of endovascular techniques more desirable. The success rate of embolization varies between 18 and 92%,3,13,14,15,16,17,18,19,20 21 reflecting the different complexity of the treated lesions as well as different definitions of technical and clinical success and variations in technique inherent to the variations between operators and centers. In our series we report a clinical success rate, defined as a decrease in the Schöbinger stage, of 77.8%. The number of interventions per patient was significantly higher in Yakes type IV AVMs, which is in line with the results reported by Griauzde J. et al.22 Several medications have been tried for the treatment of infiltrative AVMs, targeting different angiogenesis and vascular remodeling pathways, which seem to play a crucial role on their pathophysiology.23,24 Six major pharmaceutical classes have been used as off-label therapy based on this rational: 1) Thalidomide, 2) mTOR acting drugs (Rapamycin/Sirolimus and derivatives), 3) Matrix-Metallo-Proteinase-Inhibitors (Doxycycline, Marimastat and others), 4) Selective VEGF pathway inhibitors (Bevacizumab and others), 5) в-adrenergic inhibitors (Propranolol) and 6) Interferon.4,23-25 More recently, the MEK inhibitor (trametinib) has also been successfully used, based on the recent association between AVMs and MAP2K1 mutations.25,26 All of these therapies have variable results and none of them has yet been approved as current therapy. However, they appear, in our perspective, as potential adjuvants to the endovascular therapy and may reduce the progression and therefore the number of interventions in high-risk patients, such as the Yakes type IV AVMs.
Tissue necrosis was reported in two patients, requiring toe amputation in one of them. Though, the previous embolization procedures culminated with the involution of the lesion and allowed us to perform a minor amputation to control the patient’s symptoms and significantly improved her quality of life.
Although the reduced number of patients included do not allow the extrapolation of large-scale conclusions, the results obtained were satisfactory both in terms of efficacy and safety.
Conclusion
Endovascular treatment of arteriovenous malformations through nidus and/or afferent/efferent embolization requires a detailed angiographic characterization. Still, it seems to be an effective therapeutic strategy with a low risk of complications. An approach guided by the understanding of the angioarchitecture appears to optimize the results of endovascular treatment and the Yakes Classification seems to be not only an important tool for the approach’s choice but also a predictor of the clinical course and need for reintervention. However, the natural history of this pathology remains inadequately understood, curative perspectives still offer marginal results and the intervention should now be focused on preventing the destructive progression of these lesions, always requiring long-term follow-up.