Introduction
Osteoradionecrosis of the facial bones, especially of the mandible (ORNM), is a potentially debilitating complication of radiotherapy (RT) in the treatment of head and neck cancer. The clinical presentation is variable, and numerous risk factors are well-known.1
The ORNM is usually defined by the presence of exposed and non-healing irradiated bone for at least 2 to 3 months, with no evidence of tumour recurrence. Generally, it is accompanied by contiguous soft tissue necrosis.1 The mandible is the bone most frequently affected, partly due to its lower vascularisation and thinner mucosa, as well as the constant bone turnover at the level of the periodontal ligament, which is subjected to daily mechanical action and frequent dental pathology.1,2
The incidence of this complication varies between 5% and 15%, with more than 70% of cases presenting in the first 3 years after RT. In the literature, there are references to cases developed early, within 3-7 months and, at the other extreme, anecdotal cases more than 30 years after the end of RT.1
Several risk factors are described in the literature: total radiation dose (>60 Gy) and its fractionation, irradiated mandibular volume, and the RT technique; concurrent chemotherapy; tumour volume and bone infiltration; exodontia before (<21 days) or after (<2 years) RT; local biopsies, local infection and poor oral hygiene; malnutrition and alcohol and/or tobacco abuse. Age, hypertension, diabetes and connective tissue diseases appear to be predisposing factors.1,3-5
Recently, remarkable progress has been made in clarifying this entity's pathophysiology through advances in Molecular Biology and Imaging. The diagnosis is based on clinical/radiological criteria, and the therapeutic approach is multimodal.1,4
In recent decades, several therapeutic options have been considered in the prophylaxis and treatment of ORNM, including supportive therapy, ultrasound, hyperbaric oxygen therapy (HBOT), surgical resection with reconstruction and recently, the use of drugs that can reverse the radiation-induced fibroatrophic process (RIF). However, in the established ORNM, the chronic process does not solve spontaneously, and optimal management has yet to be established.1,3-5) Currently, HBOT is considered by the European Committee for Hyperbaric Medicine (ECHM) as a modality of treatment for ORNM (degree of recommendation I/ level of evidence B).3
The purpose of this article is to review the relevant literature on ORNM pathophysiology, clinical presentation and different classifications, as well as to discuss its management, with a particular focus on HBOT.
Pathophysiology
Regarding the pathophysiology of ORNM, two theories prevail.
The first was carried out by Marx et al,6 in 1983, describing the “3 H” paradigm to explain its pathogenesis. According to this theory, ionising radiation leads to peri and endarteritis, hyperaemia, hyalinisation, fibrosis and vascular thrombosis (hypovascularisation), with subsequent interference in metabolic and tissue homeostasis, which will result in decreased oxygen diffusion in the tissues (hypoxia) and cellular death (hypocellularity) (Fig. 1).
In 2004, Delanian et al,7 described the RIF as the main radio-induced mechanism. This fibroatrophic theory required that the key event for the progression of the ORNM was related to the activation and dysregulation of fibroblastic activity, leading to the formation of atrophic tissue in a previously irradiated area.
According to the work of Canney and Dean,8 the transforming growth factor-beta (TGF-β), through Smad proteins, is considered the main cytokine involved in RIF. The entire fibrotic process is initiated, enhanced and sustained by its TGF-β1 subunit. In vitro studies documented that this proinflammatory cytokine could induce fibroblast proliferation through premature expansion and differentiation from a pool of pre-fibroblastic naïve cells. During the pre-fibrotic phase, the TGF-β1 secreted by platelets initiates a cascade of events, including the recruitment/activation of macrophages and the secretion of chemotactic and mitogenic factors for fibroblasts. During the constitutive and chronic phases, circulating and myofibroblast-produced TGF-β1 contribute to the self-perpetuation of the fibrotic process. In this context, the overexpression of other inflammatory cytokines will favour the proliferation of smooth muscle tissue and collagen production by fibroblasts, thus initiating the fibrosis process. The expression and activity of these different cytokines and growth factors (e.g., thrombin, IL-1, IL-4, IL-6, TNF-α, INFβ, IGF-1, PDGF, FGFb) vary depending on the affected tissue and the phase of RIF, with direct or indirect interference in the onset and maintenance of the fibrotic process. These factors, which are infiltrated in the stroma in different molecular forms, may be locally released subsequently from the receptors of this cellular matrix, allowing persistent local inflammatory stimulation (Fig. 1).1,4-5,7-10 The interaction of ionising radiation with tissues directly and transiently generates radical oxygen species (ROS) in the inflammatory focus. During the exudative phase, contact with collagen degradation products stimulates polymorphonuclear cells and macrophages, releasing supplementary ROS. It is, therefore, a self-sufficient process of chronic inflammation through which local homeostasis is disturbed.1,4
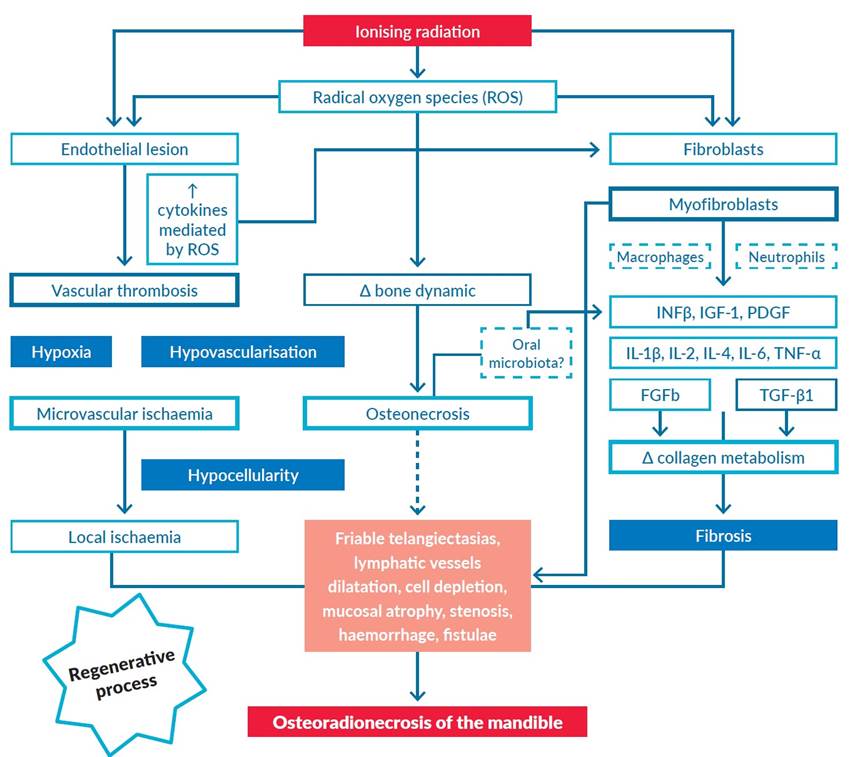
Figure 1 Representative scheme from the several pathophysiological mechanisms involved in osteoradionecrosis of the mandible: the hypoxia/hypocellularity/hypovascularisation and the radiation-induced fibroatrophic processes.
FGFb, basic fibroblast growth factor; IFN(, interferon beta; IGF1, insulin-like growth factor 1; IL, interleukin; PDGF, platelet-derived growth factor; ROS, radical oxygen species; TGF-(1, transforming growth factor beta-1; TNF-α, tumour necrosis factor-alpha.
Adapted from Costa DA et al. New perspectives on the conservative management of osteoradionecrosis of the mandible: A literature review. Head Neck. 2016;38:1708-16.(1
In addition, hypoxia interferes with the balance of species of ROS/nitrogenous radicals, causing the depletion of nitric oxide levels by increasing ROS. The ROS affect stroma degradation, leukocyte chemotaxis and phagocytosis, endothelial cell surface thrombomodulin and fibroblast activation in the extracellular compartment. In the intracellular compartment, adaptive responses to oxidative stress occur by activating genes and proteins characteristic of cellular responses to stress, acting as a trigger for processes that include DNA resistance, cell cycle arrest and secretion and growth of cytokines such as IL-1, TNF-α and PDGF. These processes can also include c-fos induction, ribosylation, activation of protein kinase C phosphorylation or induction of manganese superoxide dismutase. At last, ROS interfere with biological membranes via lipid peroxidation processes, inducing genetic modulation through transcription factors (e.g., NF-κB pathway) to the redox state of cells.1,4-5,7-10
Recently, a unique intestinal microbiota signature was identified in an animal model of radiation proctitis, amenable to increasing the expression of IL-1β, IL-6 and TNF-α (Fig. 1). Mice colonised with previously irradiated intestinal microbiota were more prone to proctitis when compared to control with naïve intestinal microbiota.11 Ionising radiation in the head and neck region appears to modify the oral cavity microbiota towards a flora associated with periodontal disease.12 Hence, is it possible to have an oral microbiota signature characteristic of the irradiated regions of the oral cavity? And if so, could these microorganisms contribute to an increased risk of ORNM?
Clinical Presentation
The clinical presentation of ORNM can vary from mild local pain, dysesthesia and halitosis to more severe symptoms, such as intractable pain, secondary infections, orocutaneous fistulas, trismus, bone exposure and pathological fracture.1,13
In the early stages of ORNM, chronic exposure of the mandibular bone exists. This devitalised exposed bone can be observed through the ulcerated and necrotic mucosa. As the disease progresses, the pain pattern may worsen and be associated with other symptoms, such as dysesthesia, paraesthesia or anaesthesia, oedema, trismus, impaired chewing/swallowing/phonation, local infection and increased predisposition to bacteraemia, halitosis, dysgeusia and impaction of food in the area of bone sequestration. In the most severe stages, patients may present with fistulisation of the oral mucosa or skin, complete bone necrosis and pathological fracture. In addition, spontaneous osteomyelitis, cellulitis, and risk of bleeding may occur.1,4,5,13,14
Table 1 is intended to be a summary of the different classifications of ORNM that have been proposed in the literature.6,15-23
Table 1 Classification of stages/grades of osteoradionecrosis of the mandible.
| Reference | Stages/Grades | Description |
| Marx6) (1983) | I-III | |
| Coffin et al.15 (1983) | Minor-Major | Based on clinical and imaging findings: Minor: A series of small sequestra which separate spontaneously after varying periods of weeks or months. These areas cannot be demonstrated radiologically. Major: Necrosis occurring to an extent that involves the entire thickness of the mandible, and a pathological fracture is inevitable. This form can be obviously seen radiologically. |
| Morton and Simpson16 (1986) | Minor-Major | Based on clinical and response to treatment: Minor: Ulceration with exposed bone and a history of bony spicules that healed spontaneously over a period of months. Moderate: Exposed bone and small sequestra limited in nature and healing spontaneously with conservative treatment within 6-12 months. Major: Large areas of exposed bone, with formation of large sequestra, possible fracture and sinus formation. These cases often require radical treatment. |
| Epstein et al.17 (1987) | I-III Subcategories: a) No pathological fracture b) With pathological fracture | Disease progression: I: Resolved, healed. II: Chronic (>3 months), persistent non-progressive. III: Active, progressive. |
| Glanzmann and Gratz18 (1995) | 1-5 | Duration of bone exposure and treatment necessity: Stage 1: Bone exposure without signs of infection and persisting for at least 3 months. Stage 2: Bone exposure with signs of infection or sequester and with no signs of grades 3-5. Stage 3: Bone necrosis treated with mandibular resection with a satisfactory result. Stage 4: Bone necrosis with persisting problems despite mandibular resection. Stage 5: Fatal toxicity. |
| Clayman19 (1997) | I-II | Based on clinical findings: I: Bone lysis occurs under intact gingiva or mucosa. II: A more aggressive type in which soft tissues break down, exposing the bone to saliva, and causing secondary contamination. |
| Store and Boysen20 (2000) | 0-3 | Based on clinical and imaging findings: 0: Mucosal defects only. 1: Radiological evidence of necrotic bone with intact mucosa. 2: Positive radiological findings with denuded bone intra-orally. 3: Clinically exposed radionecrotic bone, verified by imaging techniques, along with skin fistulae and infection. |
| Schwartz and Kagan21 (2002) | I-III | Based on clinical and imaging findings: I: Minimal soft-tissue ulceration and limited exposed cortical bone. Patients are treated with conservative management. II: Localised involvement of the mandibular cortex and underlying medullary bone. IIa: Minimal soft tissue ulceration. IIb: Presence of an orocutaneous fistula and mild soft-tissue necrosis. III: Full-thickness involvement of the bone, including the inferior border. Pathological fractures may also be present. |
| Notani et al.22 (2003) | I-III | Based on clinical and imaging findings: I: Confined to the alveolar bone. II: Limited to the alveolar bone and/or the mandible above the level of the inferior alveolar canal. III: Extended to mandible below the level of the inferior alveolar canal and/or skin fistulae and/or pathological fracture. |
| NCI CTCAE* version 5.023 (2017) * National Cancer Institute Common Terminology Criteria for Adverse Events | 1-5 | Based on clinical findings: 1: Asymptomatic; clinical or diagnostic observations only; intervention not indicated. 2. Symptomatic; medical intervention indicated (e.g., topical agents); limiting instrumental ADL. 3. Severe symptoms; limiting self-care ADL; elective operative intervention indicated. 4. Life-threatening consequences; urgent intervention indicated. 5: Fatal toxicity. |
Treatment
The most critical aspect of reducing the incidence of ORNM is the prevention of local trauma, such as exodontia and placement of dental implants. Therefore, adequate dental care must be performed before starting RT. Care for daily oral hygiene, as well as alcohol and smoking abstinence, are also fundamental in this process (Fig. 2).1,4,5,13
Regarding therapeutic management, there is some controversy. Treatment is multimodal, including conservative early-stage measures and surgical resections with reconstruction for advanced or refractory stages.1,4,5,13
Based on the complex pathophysiology of ORNM, different complementary treatments have been proposed (Fig. 2).1,4
According to the classical theory of radiation, trauma, and infection, medical treatment would undergo long cycles of antibiotics and debridement. In case of insufficient local control, mandibulectomy would be the only option.1 Marx et al,24) based on the theory of hypoxia, documented that the exposure of irradiated tissue to HBOT would induce fibroplasia and angiogenesis. Some authors advocated ultrasound treatments with frequencies of 3 MHz during 10-15 min/day for 40-100 days to promote angiogenesis, although the results are disparate in the literature.1,5,25 Corticosteroid therapy has shown utility in reducing the acute inflammatory reaction associated with fibrosis, although ineffective in reversing the RIF.1,26 Pearlman et al studied osteomyocutaneous grafts for mandibular reconstruction in irradiated tissue. Later, microvascular flap grafting techniques were used to reconstruct mandible deformities after surgery and RT.1,5,13
Recently, new therapeutic protocols have been developed based on the potential for reversion of the proinflammatory myofibroblastic pathological phenotype. In 1998, the combination of tocopherol (vitamin E) with pentoxifylline was clinically tested for 18 months of treatment, with antifibrotic action being demonstrated in the superficial and deep cervicothoracic region.27 Tocopherol partially inhibits TGF-β1 and has antioxidant potential. Pentoxifylline demonstrated significant effects on the modulation of the vascular response, anti-inflammatory (e.g., anti-TNF-α) and antioxidant, with an impact on fibroblast inhibition and extracellular matrix proliferation.1,7,27,28) However, the evidence for pentoxifylline monotherapy seems contradictory in treating RIF, with a possible rebound effect after its suspension, suggesting that the optimal treatment duration should not be less than 12 months.1,5 One year after this pioneering report, Delanian et al29 conducted a phase II clinical trial that combined tocopherol 1000 IU/day with pentoxifylline 800 mg/day in patients with superficial RIF, previously irradiated for head/neck and breast neoplasms. The mean regression rate for surface RIF was 53%, and the SOMA scores (subjective, objective, medical management, and analytical evaluation of the lesion) were 66% and 48% at 6 and 12 months. In 2011, Delanian et al30 published a phase II trial (PENTOCLO) in which the association of this doublet with clodronate was tested in refractory ORNM, with a poor prognosis. The main objective of this study was to determine the maximum efficacy and the optimal duration of treatment until the complete resolution of the ORNM. Unlike the latest generation of bisphosphonates, oral clodronate directly affects osteoblastic cells, increasing bone synthesis and decreasing osteoclastic activity. In addition to this inhibitory effect on bone resorption, an anti-inflammatory action has also been described.1,30) Over 8 years, patients underwent the following oral therapeutic protocol: tocopherol 1000 IU/day, pentoxifylline 800 mg/day and clodronate 1600 mg/day (weekdays) alternating on weekends with 20 mg of prednisolone and 1000 mg ciprofloxacin. The duration of treatment and the dosages were based on previous pharmacokinetic data and experience in treating approximately 1000 patients with RIF. The prolonged treatment protocol (16 +/- 9 months) was safe and well-tolerated. All patients improved, with an exponential reduction in exposed bone depending on the time course (2 months: 42%; 4 months: 62%; 6 months 77%; 12 months 92% and 18 months 96%). This medicative therapy was combined with sequestrectomies in 36 of the 54 patients (66.7%). Clinical improvement was determined regarding suspension of analgesics, absence of new fractures, closure of cutaneous fistulas and radiological evolution: SOMA: 6 months 64%; 12 months 89%; 30 months 96%.30
This triplet therapy bases its clinical benefit on the potential to reverse the RIF. Thus, it interferes decisively at several levels, namely: inflammation (anti-TNF-α effect, inhibition of TGF-β1 and other proinflammatory cytokines), oxidative stress (elimination of ROS, protection against lipid peroxidation of the membrane), extracellular matrix (inhibition of fibroblast proliferation and increased collagenase activity), bone resorption (reduced activity and the number of osteoclasts and increased bone synthesis by osteoblasts) and vasodilation/ deformability of erythrocytes.1,30
Hyperbaric Oxygen Therapy
The HBOT is a treatment based on inhaling pure oxygen (100%) in an environment with higher atmospheric pressure than at sea level (1 atmosphere absolute - ATA). The HBOT sessions are held inside hermetically sealed hyperbaric chambers, classified as type IIb medical devices (Directive 93/42 ECC of June 14, 1993, concerning medical devices). This treatment is used in several clinical conditions as well as in professional and military training. Therapeutic HBOT usually involves pressures higher than 1.4 ATA (141.8 kPa), frequently ranging between 2.0 (202.6 kPa) and 2.5 ATA (253.3 kPa) for 60 to 120 minutes (min).31
Furthermore, HBOT increases the partial pressure of oxygen in the tissues, promoting a high availability of oxygen and subsequent stimulation of fibroblast proliferation and collagen synthesis, osteoblastic activity in irradiated areas, angiogenesis and reepithelialisation, and the bactericidal/bacteriostatic effect, as well as synergism with some antibiotics in the treatment of local infections of the oral cavity.1,32,33
In 1976, Hart and Mainous34 suggested that the beneficial action of HBOT was due to the angiogenic and cell proliferation effects. Marx et al35 published experimental work on an animal model (rabbit) in which, after 20 sessions of HBOT (2.4 ATA, 90 minutes daily), was observed increase of 8 to 9 times more in vascular density compared to controls under normobaric oxygen or air-breathing (p-value= 0.001). In 1983, the same author was also the first to systematically describe the use of HBOT in treating human ORNM.6 At that time, it was defined as 3 stages according to the response to 30 initial HBOT sessions (2.4 ATA, 90 minutes, 5 times a week). With this treatment protocol, ORNM resolution was achieved in 15% of cases in stage I, 14% in stage II and 70% in stage III (n = 58 patients) (Table 1).6 In another study, Marx and Johnson36 performed repeated biopsies before and after 20 sessions of HBOT (2.4 ATA, 90 minutes daily) in a subgroup of 50 patients with ORNM. They noted an increase in the number of apparently functional capillaries and fibroblast cells. In 1997, Thorn et al37 determined that the partial pressure of oxygen at the level of the irradiated gingiva increased from an average of 20.4 [16.6-23.2] mmHg to 34.7 [27.8-40, 0] mmHg after 30 sessions of HBOT (2.4 ATA, 90 minutes, 5 times a week). Marx et al,24 in another prospective study that compared HBOT to penicillin in the prophylactic context of ORNM, also demonstrated the increase and long-term maintenance of high transcutaneous oxygen tension in the irradiated tissue after 20 sessions of HBOT (2.4 ATA, 90 minutes, 5 to 6 times a week). In 1998, London et al,38 using a therapeutic protocol practically similar to that of Marx, demonstrated clinical benefit in all 16 patients in the study with improved pain patterns. Marx and Ames39 also reported the efficacy of HBOT on the viability of osteomyocutaneous grafts in previously irradiated tissues with a success rate of 91.6%. In 2000, Maier et al40) carried out a study with 41 patients with advanced ORNM (Marx stage III) treated with or without postoperative HBOT (all submitted to debridement and antibiotics) with a success rate of 65%. In 2002, Feldmeier and Hampson41 published a systematic review analysing 14 studies and 423 patients, with an overall response rate to the treatment of 83.6%.
In 2004, Annane et al42 published the results of the only multicentre, randomised, double-blind, placebo-controlled study (with a gas mixture of 9% oxygen and 91% nitrogen at 2.4 ATA, equivalent to 1 ATA versus HBOT at 2.4 ATA in the experimental group). The data presented did not reveal - as expected - a beneficial clinical effect of using HBOT in treating ORNM. However, some criticisms were made (including in a Cochrane review)1,5,43,44) to this trial, namely:
The accepted standard treatment for HBOT in ORNM was not followed, having been considered an atypical regimen, with 2 daily HBOT sessions for a total of 25 sessions, and there was also no uniform and precise indication for surgical debridement of unviable bone tissue after performing HBOT, contrary to what is reflected in the usual clinical protocols;
One of the definitions of therapeutic failure determined in this trial was the need for surgery in the subgroup of patients with less severe ORNM when it is part of the multimodal therapeutic approach;
Severe forms of ORNM that include mandibular fracture and bone resorption of its lower border were not considered, which makes its generalisation difficult for these patients;
Most patients who have not recovered within 1 year would have already demonstrated the absence of a proven benefit from previous therapies.
In 2009, Freiberger et al,45 based on the multimodal therapeutic approach described by other authors such as Marx6,24,35,36,39 and Curi et al,46 reported an overall response rate of 88% (57/65 patients) that remained stable during the follow-up period with an average duration of 86.1 [64.0-108.2] months in non-smokers (n= 20) versus 15.8 [8.4-23.2] months in smokers (n= 14). Ideally, the initial therapeutic protocol would comprise 30 sessions of HBOT before and 10 after surgery. However, the median of HBOT sessions was 39 [19-55] since not all patients underwent new treatments after the surgical procedure. No correlation was established between the total number of HBOT sessions and the efficacy of this treatment. Another relevant fact of this study was that 72% of patients who improved the ORNM with HBOT had previously been treated with at least 3 months of conservative treatment. On the other hand, some studies have not revealed the clinical benefit of HBOT as a complementary treatment with surgery.47
In 2012, an 8-year prospective follow-up study of 43 patients with ORNM was published by Hampson et al,48 in which an overall response rate of 94% was observed after an average of 40 [30-60] HBOT sessions (2.4 ATA, 90 minutes, 5 times a week). In 2015, Tahir et al,49 in the largest study carried out in Australasia, aimed to assess the efficacy of HBOT in radio-induced lesions. In relation to the established ORNM, necrosis of the soft tissues of the head and neck and xerostomia, the overall response rate to HBOT was 86%, 85% and 64%, respectively. The HBOT protocol for these and other radio-induced lesions was similar (2.4 ATA, 70 minutes, 7 times a week), except for the number of sessions that varied depending on the clinical indication.
In 2016, Bennett et al,50 in the updated Cochrane meta-analysis50 on the efficacy and safety of HBOT in treating radio-induced lesions. They reported evidence of moderate quality for the process of reepithelialisation and closure of the mucosa contained in the ORNM [risk ratio (RR) of 1.3; 95% confidence interval (CI) 1.1-1.6; p-value= 0.003, number needed to treat (NNT) for an additional benefit (NNTB) 5; 246 patients, 3 studies] and for a lesser probability of cure in the postoperative period of ORNM, in case of omission of adjuvant HBOT (RR 4.2; 95% CI 1.1-16.8, p= 0.04, NNTB 4; 264 patients, 2 studies) The analysis of isolated studies highlights the significant increase in the probability of improvement or cure with HBOT after hemimandibulectomy (RR 1.4; 95% CI 1.1-1.8, p= 0.001, NNTB 5) or flap surgery (RR 8.7; 95% CI 2.7-27.5, p= 0.0002, NNTB 4). In patients undergoing HBOT, there was also an improvement in the probability of healing irradiated dental alveoli after tooth extraction (RR 1.4; 95% CI 1.1-1.7, p= 0.009, NNTB 4).
The more recent HOPON randomised controlled trial, conducted by Richard J. Shaw et al (2019),51 challenges the long-standing practice of using HBOT to prevent ORNM following high-risk surgical procedures in irradiated mandibles. All patients received chlorhexidine mouthwash and antibiotics. In the HBOT group, patients underwent 30 HBOT sessions at 2.4 ATA, with each session lasting 70 minutes, seven times a week. The trial's primary endpoint was the incidence of ORNM at 6 months, and the results showed no significant difference between the HBOT and control groups (OR 1.13; 95% CI 0.14-8.92, p=1). However, patients in the HBOT group experienced milder acute symptoms, including reduced pain, swelling, bleeding, improved mouth opening, and easier eating.
In 2022, Lone E. Forner et al52 conducted a study that combined data from two randomised clinical trials, DAHANCA-21 and NWHHT2009-1, to assess the impact of HBOT on ORNM. Patients with ORNM requiring surgical intervention were divided into two groups: group 1 received surgical necrotic bone removal in conjunction with pre and postoperative HBOT sessions (n=30), while group 2 underwent surgical bone removal alone (n=35). One year after surgery, the healing rate in group 1 was 70%, compared to 51% in group 2 (OR 2.2; 95% CI 0.7-7.0, p=0.13). However, the effect of HBOT did not reach statistical significance, likely due to being underpowered and, therefore, susceptible to type II error.
A pilot clinical trial53 is underway to compare HBOT (Marx's protocol)6 to medical treatment with pentoxifylline, tocopherol +/- clodronate (based on the PENTOCLO therapeutic regimen)30 in 16 patients with ORNM.
Currently, HBOT is considered by ECHM as a potential prophylactic and therapeutic intervention for ORNM (degree of recommendation I/ level of evidence B).3
Herein, we report the relevant literature review on the pathophysiology of ORNM, clinical presentation and different classifications, as well as discuss its management, including a proposal for a therapeutic algorithm, which takes into account the variables related to the patient, institution, and the severity of the clinical context (Fig. 2).
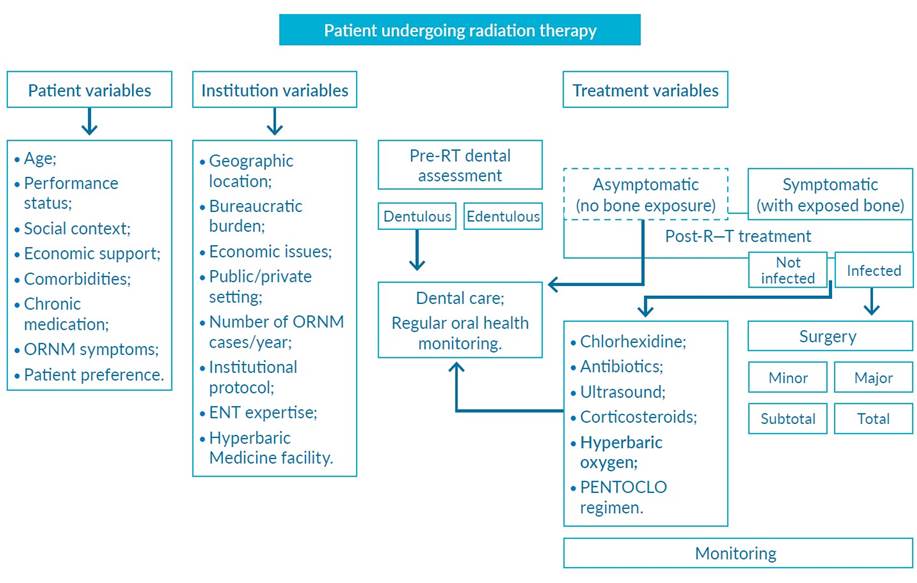
Figure 2 Suggested treatment algorithm for osteoradionecrosis of the mandible based on variables related to the patient, institution, and the severity of the clinical context. ENT, otorhinolaryngology; ORNM, osteoradionecrosis of the mandible; PENTOCLO regimen, tocopherol 1000 IU/day, pentoxifylline 800 mg/day and clodronate 1600 mg/day (weekdays) alternating on weekends with 20 mg of prednisolone and 1000 mg ciprofloxacin; RT, radiotherapy. Adapted from Costa DA et al.
Conclusion
One can conclude that there are several therapeutic strategies for this clinical condition, but gold standard management has yet to be established. The HBOT is often combined with surgery according to Marx´s protocol or to prevent ORNM when dental extractions or implant placement are considered. The most conservative treatment aims to decrease or delay the need for mandibular surgery and improve symptomatic control and quality of life. On the other hand, major surgical intervention should be considered in severe refractory disease with complications.
The authors expect that, shortly, the controversy regarding HBOT in ORNM will be dimmed. Further prospective, randomised studies are needed to better validate the effectiveness of HBOT in the 'real world' clinical practice, including the results of the prospective study comparing HBOT to medicative therapy with the PENTOCLO regimen.53















