Introduction
The study of sinonasal papillomas (SPs), which are benign epithelial neoplasms, is of significant interest due to their aggressive behavior, predisposition to recurrence and risk of malignancy.1 Despite being considered benign neoplasms, their biological behavior confers them aggressive characteristics, leading them to invade structures adjacent to the nose and paranasal sinuses, even in the absence of associated malignancy. 2 Timely clinical diagnosis of these neoplasms is a complex task, considering that they are rarely discovered in their early stages and that late diagnosis leads to more challenging treatment options and a worse prognosis. 3
Adequate clinical management of these neoplasms includes early diagnosis, complete surgical excision, and rigorous postoperative follow-up. 4 Even tough these neoplasms are considered benign, their biological behavior, as noted above, can be very worrisome, even in the absence of associated malignancy, because these tumors grow in a narrow anatomical space, surrounded by critical organs such as the brain and the orbital cavities. 4 SPs are usually asymptomatic in their early stages. Therefore, their early detection is fundamental. Their clinical presentation is usually unilateral and asymptomatic in the early stages of growth. Symptoms begin to appear only after the tumor has reached a significant volume and can cause nasal obstruction, nasal secretions, bleeding, headaches, smell alterations and ophthalmologic and audiologic manifestations. 5
Based on the general endoscopic, imaging and histopathological diagnostic evidence commonly observed in SPs, it is crucial to determine when a complementary study should be indicated to facilitate and clarify the timely diagnosis of these neoplasms. 6 This may allow a higher rate of early diagnosis to favor prompt treatment with a better evolution and prognosis for the patient. 4
The endoscopic, imaging and histopathological assessment of SPs play an essential role in the diagnostic task of SPs, and these have been studied in detail on a case-by case basis. However, establishing a high clinical Index of Suspicion (IS) for these lesions and the close correlative interrelationship of all the studies mentioned above, has not been analyzed in depth and together may be relevant to confirm or dismiss the definitive diagnosis of these neoplasms. 7
Histopathologically, three subtypes of SPs are distinguished: Inverted Papillomas (IPs), Fungiform or Exophytic Papillomas (FPs) and Columnar Cell Papillomas or Oncocytic (OPs). Although the histopathological diagnosis of these neoplasms is one of the key aspects in the final diagnosis of these lesions, it is not free of inconveniences derived from the sampling, examination and interpretation of the tumor specimen by the pathologist. 2
Knowledge about the etiology, clinical features, behavior and evolution of SPs has been influencing the state of the art in the diagnosis, management and treatment of SPs, especially in its early diagnosis and early treatment and the complex management scenarios that may arise in cases of advanced neoplasms of this disease in its advanced stages. 1
The present work focuses on the early identification of SPs based on the formulation of a Suspicion Index Scale (SIS) based on endoscopic and imaging evidence commonly observed in SPs for adequate surgery planning.
Purpose of this study
To design and evaluate the effectiveness of a quantitative and correlative Suspicion Index Scale (SIS), based on endoscopic, imaging and histopathological findings commonly observed in SPs from the establishing of an IS, to build a scale based on different findings commonly observed in these neoplasms.
Materials and methods
A retrospective, descriptive, longitudinal, quantitative and correlational investigation of 103 patients diagnosed with SPs, evaluated and treated from 1993 to June 2021 was done. The selected population sample was obtained from a database of 27,416 medical records reviewed.
Patients were informed, orally and in writing, about the research objectives and this provision was formalized in the respective informed consent forms. The inclusion criteria considered in this investigation were: patients with a confirmed diagnosis of SPs studied endoscopically, through imaging with computed tomography (CT) of the paranasal sinuses and magnetic resonance imaging (MRI) and histologically. Patients underwent surgery and had a minimum follow-up of 5 years after surgery. Exclusion criteria included those patients with suspected or confirmed diagnoses of SPs who voluntarily discontinued their follow-up and patients who did not approve the informed consent.
Once the selection of the patient population and the type of research applied were defined, the incidence of SPs was evaluated according to sex and age, clinical manifestations, time evolution, and endoscopic and imaging findings.
This research was carried out in a private third-level referral Otorhinolaryngology Center in Caracas, Venezuela, and approved by the Ethics Committee of the Institutional Review Board. This study adhered to the bioethical principles established in the World Medical Association's Declaration of Helsinki and had no external source of funding or conflict of interest.
Statistical analysis:
The statistical analysis of variables studied was summarized, calculated and evaluated with Microsoft Excel 2010 and IBM® SPSS®, which allowed the collection of absolute, relative and percentage frequencies and measures of central tendency to determine the diagnostic efficiency of the studies performed in patients with SPs through measures of sensitivity, specificity, predictive values, and the rate of false positives and negatives recorded. In addition, the relative levels of statistical contingency (Fisher's test), diagnostic coincidence proportions (Cohen's kappa coefficients) and the levels of diagnostic accuracy and the reliability of SPs according to IS levels (Cohen's d test) were also determined.
Results
On a database of 27,416 medical records reviewed, 1,897 cases of patients with the finding of nasal polyposis were selected, of which 103 patients were identified with the histopathological diagnosis of SPs. Patients with these neoplasms represented 0.4% of the total number of clinical histories analyzed. The male/female ratio was 77%/23%, The ages of the affected patients ranged from 9 to 80 years, with the most affected group being patients between 51 and 60 years.
The clinical manifestations and nasal endoscopy, imaging and histopathology findings observed in all patients were analyzed correlatively. The most referred symptom was nasal obstruction in 91% of the patients. In 78%, this nasal obstruction was reported as unilateral, and in 22% of cases, nasal obstruction was bilateral. Rhinorrhea was present in 63% of cases, headache in 34%, hearing loss and epiphora in 33% and 21%, respectively. Furthermore, other symptoms such as epistaxis in 10% of the cases studied, anosmia in 9% of the patients and alteration of the external aspect of the face, with deformation of the nasofacial region in 3%. Nine percent (9%) of the patients studied did not present any symptoms and the findings of lesions were incidental.
Endoscopic examination of the nasal cavities revealed in all patients the presence of a lobulated polypoid lesion of different sizes. Ninety-nine (99%) percent of these patients had unilateral lesions, and in one patient, lesions were observed on both sides. In 63% retained secretions were observed and in 13% evidence of blood in the affected nasal cavity was also observed.
The presence of a polypoid type lesion, however small, may be a signal that should arouse an IS of neoplasia within the nasal cavity, despite the fact that, in most cases, this finding could be related to inflammatory processes. Based on this suspicion criteria, other complementary studies, which may be important to clarify the diagnosis of an early neoplasm, may be indicated. (Figure 1)
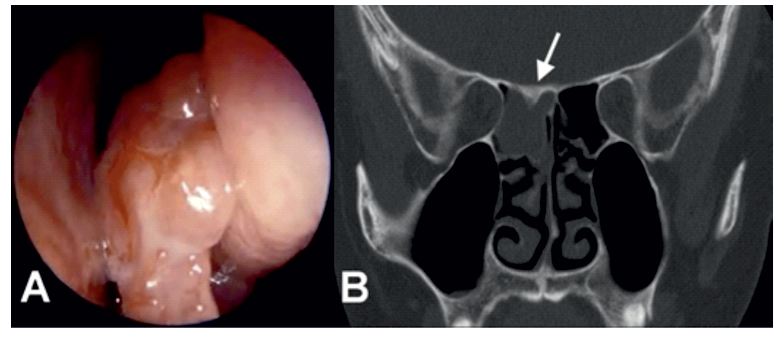
Figure 1: (A) Nasal endoscopic view of polypoid mass with a lobulated morphology pedicled on the right middle meatus. (B) Coronal CT image in the same a patient showing complete opacification of with inverted papilloma shows localized hyperostosis of the superior wall of the posterior ethmoid sinus (white arrow).
All patients were evaluated radiologically through a bimodal diagnostic approach of imaging studies, through a sinus CT scan and a contrast-enhanced MRI. The combination of both studies made it possible to correlate the images obtained and their respective correspondence with the information collected in the endoscopic evaluation performed at the initial consultation. Imaging studies identified different findings commonly observed in patients with SPs, such as opacification, focal hyperostosis and bone erosion by CT scan and images with a contoured cerebriform pattern and extra-sinonasal extension by contrast-enhanced MRI.
In 91% of the CT scans of patients with SPs, images with different degrees of opacification were observed, coinciding with the polypoid lesion observed endoscopically. The opacification images observed were present in 60% of the cases on the right side, in 39% of them on the left side and in 1% on both sides. Opacification tomographic images indicated the anatomic location and extent of the pathologic process in the paranasal sinus cavities. The anatomical involvement partially involved one nasal cavity and one paranasal sinus in 11% of the cases. In 35% of patients, the involvement affected the entire nasal cavity and one paranasal sinus. In 26% of the cases, the opacification involved an entire nasal cavity and two paranasal sinuses. In 19% of the patients this finding affected the entire nasal fossa and more than two paranasal sinuses and/or in 1% of the cases opacification was identified on both anatomical sides.
Focal hyperostosis, considered a tomographic sign predictive of the area of anatomic adhesion and the site of origin of the SPs, was identified in 79% of the cases. This finding was observed in the lateral nasal wall in 32% of patients, within the maxillary sinus in 15% of cases, in the anterior ethmoidal sinus in 12%, in the posterior and frontal ethmoidal sinuses in 2% of cases and the sphenoidal sinus in 5% of cases. Focal hyperostosis was located in 12% of the assessed patients in two or more anatomical walls. (Figure 2)
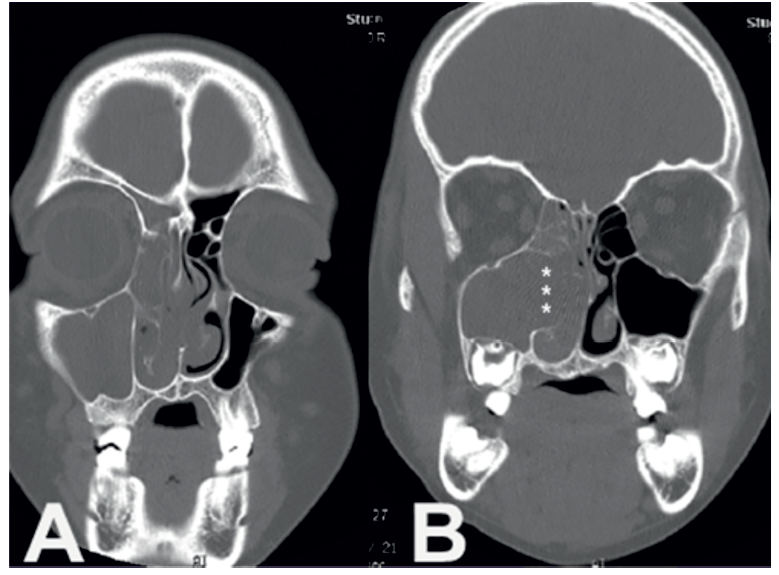
Figure 2: Sequence of Coronal computed tomography scan in a patient with a right polypoid mass with a complete homolateral opacification of maxillary and ethmoidal sinus which corresponded to an inverted papilloma. (B) Note the area of bony erosion on the middle maxillary sinus wall (white asterisks) due to mass expansion arising from the left metatal region and extending toward the homolateral nasal cavity.
The CT scan showed signs of erosion and destruction of bone walls in 84% of the patients studied. Bone involvement involved non-critical bone walls (septum, turbinates, lateral wall) in 69% of the cases and critical bone walls (orbit, skull base) in 16% of the patients. In patients in whom erosion of critical bone walls was found, in 9% of cases, this alteration affected the orbital wall, in 6% the skull base, and in 1% of cases, this alteration affected both anatomical walls.
In 86% of the cases studied, T1-weighted MRI sequences without contrast and T2-weighted MRI sequences with contrast revealed the presence of images with signs of hypo- and hyperintensity in the interior of the neoplasms, known as "contoured cerebriform pattern". The MRI images also indicated that in 16% of these cases, there were noticeable signs of neoplastic progression and extra-nasosinusal extension. The correlative comparison of the CT scan and MRI of this group of patients showed consistency with the presence of advanced disease. (Figure 3)
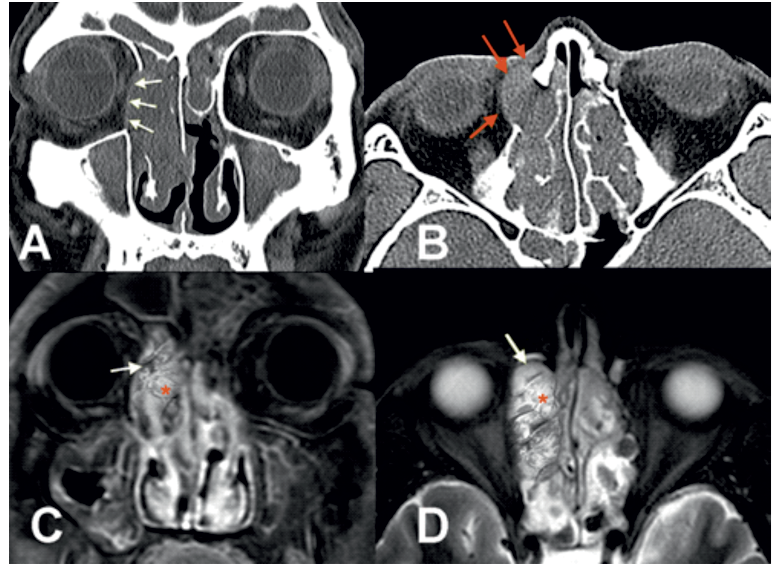
Figure 3: CT and MRI studies with and without contrast in a patient with an IP with intraorbItal extension and soft tissues of the nasofacial region. Coronal and axial sinus CT scan (A) (B) showing a bilateral sinus opacity, with erosion of the right orbital wall (white arrows) with lateral and anterior displacement of the intraorbital content and subcutaneous cellular tissue in the nasofacial region (red arrows). Coronal and axial images on MRI in T1-weighted (C) shows a hypointense mass occupying the right nasal cavity, middle meatus and ethmoid sinus. Axial projection in T2-weighted (D), shows that the mass mainly has a high-intensity signal, Both images of MRI notably demonstrates a pattern alternating lines of high (red asterisk) and low signal intensity (white arrows) inside the mass, referred to as convoluted cerebriform pattern.
The endoscopic and imaging findings observed allowed us to assess the location, size, and extension of the neoplasms for their corresponding staging, using the scale proposed by Krouse 1. According to this scale, 31% of the patients studied were placed in Stage I of the disease, 28% in Stage II, 25% in Stage III and 16% in Stage IV. Staging made it possible for us to determine the degree of limited disease or extended disease in the nasal sinus cavities.
All patients underwent surgery, and their lesions were resected endoscopically. Intraoperatively, the insertion site of the SPs was identified in different anatomical areas, with a predictive coincidence of 67% with respect to the finding of focal hyperostosis confirmed in the preoperative CT scans.
Histopathologic findings reported were 67% IPs, 30% FPs and 3% OPs. Associated histologic malignancy (squamous cell carcinoma) related to recurrent IPs was reported in 3% of patients. The incidence of recurrent disease was observed in 33% of the group. Review of previously resected specimens for this group of patients showed a false negative rate of 24%. In 9% of recurrent cases there were no reports or material from the previous histopathologic study. The level of consistency of accurate histopathologic reports in cases of recurrent disease was 67%.
Following the histopathological confirmation of the SPs, the different endoscopic and imaging findings were summarized, tabulated, and correlated to calculate and analyze the levels of prediction, reliability, and statistical agreement and to measure the degree of confidence in obtaining a correct diagnosis of SP through the diagnosis in the studies performed. The levels of predictive assessment, significance and statistical agreement found were optimal in all patients with SPs with endoscopically identified polypoid lesion findings, revealing good results for each of the imaging findings evaluated.
The degree of diagnostic accuracy of each of the findings identified by endoscopy and imaging in patients with SPs, based on the levels of sensitivity and positive predictive values recorded, allowed for an ideal and strong correlation, with an overall mean average of 88%.(Table 1)
Table 1: Prediction, reliability and statistical agreement measures of each of the commonly findings observed in SPs.
| Search | Finding | Patients with positive findings | Patients with negative findings | Sensitivity Specificity | PV* + PV* - | True negative Index | Fisher test (p value) | 𝜿 Cohen |
| Nasal endoscopy | Lobulated polypoid mass | 100% (n=103) | 0% (n=0) | 100% | 100% | 0 | < 0.00001 | 1 |
| Imaging (CT scan) | Sinonasal Opacification | 91% (n=91) | 9% (n=9) | 91% | 91% | 9% | < 0.00001 | 0.82 |
| Focal hyperostosis | 79% (n=81) | 21% (n=22) | 79% | 79% | 12% | < 0.00001 | 0.62 | |
| Bony wall erosion | 84% (n=71 + 16) | 16% (n=16) | 84% | 84% | 16% | < 0.00001 | 0.67 | |
| Imaging (MRI) | Convoluted cerebriform pattern | 86% (n=89) | 14% (n=14) | 86% | 86% | 7% | < 0.00001 | 0.73 |
A positive correlation was observed from the initial finding of an endonasal polypoid lesion and the establishment of a high clinical IS when correlated with the imaging findings observed in patients with SPs.
The detailed analysis of each of the correlative comparisons found in patients with SPs revealed excellent levels of prediction, reliability and effect on the influence level of diagnostic probability in all contingency associations proposed. The influence levels of diagnostic probability in all comparisons made through the Cohen d Test yielded coefficients showing a high effect on the influence of diagnostic probability. (Table 2)
Table 2: Prediction, reliability and influence level measures of diagnostic probability in patients with SPs after considering a clinical high index of suspicion related to the initial evidence of an endonasal polypoid mass. This was correlated with each of the imaging findings observed in these patients (CT scan and contrast-enhanced MRI). Coefficients of diagnostic probability estimates in all correlative comparisons show coefficients that reveal a high effect of diagnostic efficiency in the patients with SPs in our investigation
| Endoscopic and imaging findings | Patients with positive findings | Patients with negative findings | Sensitivity Specificity | True negative Rate | μ | σ | Test T | Fisher test |
| Cohen d | (p value) | |||||||
| Lobulated polypoid mass | 103 | 0 | 91% | 9% | 51.1 | 72.83 | 0,66 | < 0.00001 |
| Opacification (CT scan) | 91 | 9 | 97 | 66.47 | ||||
| Lobulated polypoid mass | 103 | 0 | 78.6% | 21% | 51.1 | 72.83 | 0.78 | < 0.00001 |
| Focal hyperstosis (CT scan) | 81 | 22 | 92 | 15.55 | ||||
| Lobulated polypoid mass | 103 | 0 | 84% | 16% | 51.1 | 72.83 | 0.84 | < 0.00001 |
| Bony wall erosion (CT scan) | 87 | 16 | 95 | 11.31 | ||||
| Lobulated polypoid mass | 103 | 0 | 86.4% | 14% | 51.1 | 72.83 | 0.86 | < 0.00001 |
| Convoluted cerebriform pattern (MRI) | 89 | 14 | 96 | 9.89 |
Based on the influence levels of the diagnostic probability observed in each of the diagnostic findings commonly observed in SPs, a correlative instrument called SIS was proposed, which used as clinical parameters the endoscopic and imaging evidences observed in these patients, assigning each diagnostic finding a quantitative IS score. Each of the diagnostic parameters considered was assigned an individual score of 1 point, except for the contoured cerebriform pattern with 2 points, as this finding, which is considered the most characteristic in most SPs, is unusual in other neoplasms and is absent in all inflammatory processes of the paranasal sinuses. (Table 3)
Table 3: Endoscopic and imaging criteria considered in the SIS and score earmarked for the diagnosis of SPs.
| Diagnostic endoscopic and imaging findings criteria | Score |
| Lobulated polypoid mass (nasal endoscopy) | 1 |
| Sinonasal opacification (CT scan) | 1 |
| Focal hyperostosis (CT scan) | 1 |
| Bony wall erosion (CT scan) | 1 |
| Convoluted cerebriform pattern (MRI) | 2 |
The score sum of the different parameters listed in this SIS provided a score that indicated a predictive value indicative of suspected SPs, through the three levels established in this scale (low, moderate or high) (Table 4)
Table 4: Score degrees of diagnostic presumption of SPs based on the SIS.
| Stage | Suspicion index | Score range |
| I | Low | 1 - 2 |
| II | Moderate | 3 - 4 |
| III | High | 5 - 6 |
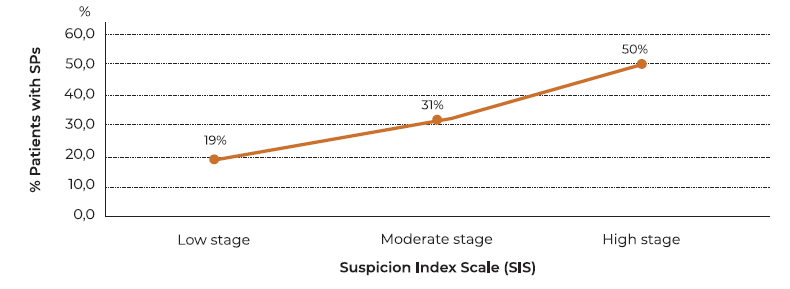
Patients with SPs were analyzed according to the IS level at which they were identified during the diagnostic process (endoscopic and imaging), allowing us to evaluate the sensitivity, specificity, predictive values and levels of probability of influence and diagnostic reliability contemplated in the scale design. Nineteen (19%) of the cases were detected with a low IS score. All patients with lesions discovered at this level coincided with early and poorly developed lesions. 31% of patients were found to have moderate IS, and 50% of patients were found to have high IS.
The estimates calculated through the Cohen's d test yielded coefficients indicating that a level of diagnostic probability strengthens the SIS to the extent that the IS levels increase, yielding a level of diagnostic superiority of low effect, in cases with low and moderate IS and medium effect in patients with high IS. Likewise, it was observed that the reliability records registered in the contingency correlations carried-out in the different degrees of SIS calculated through Fisher’s test yielded statistically significant results, which support the confidence in this instrument and in the correlative and quantitative diagnostic evaluation of the SPs. (Table 5)
Table 5: Measures of sensitivity, specificity, positive predictive values (PV+), probability of influence and diagnostic reliability according to the IS stage in which the patients were identified. Cohen's d test shows coefficients with a low effect diagnostic superiority level in cases with low IS and of medium effect in SPs with moderate and high IS. The reliability measures recorded in all the contingency correlations analyzed in the proposed scale showed statistically significant results (Fisher's test)
| Suspicion index Scale (SIS) | Patients with positive findings | Standard Error | Confidence interval for 95% | Confidence interval for 99% | Sensitivity | Patients with negative findings | Fisher test | Cohen d test |
| Positive predictive value | (p value) | |||||||
| Stage I | 19% - (n=19) | 0.03818 | 0.109 - 0.259 | 0.086 - 0.282 | 19% | 82% - (n=84) | < 0.00001 | 0.2 |
| (Low SI) | ||||||||
| Stage II | 31% - (n=32) | 0.04561 | 0.222 - 0.4 | 0.194 - 0.428 | 31% | 69% - (n=71) | < 0.00001 | 0.3 |
| (moderate SI) | ||||||||
| Stage III | 50% - (n=52) | 0.04926 | 0.408 - 0.602 | 0.378 - 0.632 | 51% | 50% - (n=51) | < 0.00001 | 0.5 |
| (High SI) |
According to the predictive levels observed in patients with SPs identified by this SIS as true positives, a curve with a progressively increasing trend is outlined, as IS levels based on correlative scoring of endoscopic and imaging parameters were increasing. (Figure 6)
When relating the quantitative degrees of IS to the different histological subtypes of SPs diagnosed, we observed that most SPs diagnosed with low and moderate IS were FPs, and most of the IPs and OPs were identified with high and moderate IS. It follows that the predictive level found in FPs according to this ISS was more significant for low and moderate IS, in the order of 17% and 13% of the cases studied. Most of these FPs were diagnosed at early and underdeveloped stages, and the recurrence observed in this type of papillomas was 4%. In addition, the majority of IPs were identified in 50% of cases with a high degree of IS, in 17% with a moderate IS and in 1% with a low IS. The recurrence disease observed in this type of papillomas was 28%. OPs, OPs, were the neoplasms less observed in our investigation and were detected at moderate and high IS levels (2% and 1%, respectively). Recurrent disease observed in this type of recurrent papillomas occurred in 1% of cases.
The feasibility of establishing a timely and optimal diagnostic correlation in identifying SPs in early stages, using the proposed ISS as a guide, has a remarkable potential, based on the levels of predictive assessment, significance, consistency and influence of diagnostic probability analyzed. (Table 6)
Discussion
Papillomas are benign neoplastic lesions which arise in any epithelial tissue. However, their presence in the nasosinusal cavities arouses a special interest due to three characteristics of these neoplasms, which differentiate them from other sinonasal neoplasms and other papillomas located particularly in other epithelia of the human body. These particular characteristics are their potential local destructive activity, their high rate of recurrence, and their risk of malignant transformation. 2
Demographic results are in accordance with the cited references. 4,5-7 Clinical diagnosis of SPs can be tricky and challenging and therefore the correlation of endoscopic and imaging findings are key in the timely diagnostic guidance of these neoplasms. However, it should be noted that most of the commonly reported findings in SPs, despite being widely analyzed individually in the literature, have occasionally been evaluated in a correlative way, through a combined bimodal approach of radiological diagnosis with CT scans and MRIs. 8-12
Although a paranasal CT scan is indeed the Gold Standard in the radiological study of chronic inflammatory and neoplastic sinus disease, because it offers the possibility of performing anatomical reconstructions using bone and soft tissue algorithms, it is essential to highlight that SPs and inflammatory sinus disease can often reveal signs on CT studies that may be common and therefore not conclusive in assuring the diagnosis of these neoplasms. This is especially relevant in cases of early or little advanced disease, since this study alone cannot specify the density limits of neoplastic tissue and differentiate the limits of soft tissue density, from a volume occupied by secretions retained within the sinus cavities. 11,13
Some authors 14,15 have pointed out that the clinical assessment of SPs should be studied through a bimodal strategy of imaging diagnosis with a CT scan and a contrast-enhanced MRI, considering the role of the latter study as a determinant in the identification of the volume of a neoplastic mass and the presence of retained secretions, as well as the coexistence of extra-sinonasal alterations, in cases of advanced tumors. 11-15
Endoscopically, SPs have a solid polypoid appearance with a lobulated morphology, which at first glance can be interpreted as inflammatory nasal polyps. An incisional biopsy performed in-office may clarify the histopathological diagnosis of a polypoid-type lesion. 16 However, some authors 17-21 have warned of a significant risk of reported false positives, ranging from 15% and 28%, because SPs can histologically coexist with fibroinflammatory polyps.
Establishing a high IS at this point prompts the performance of complementary diagnostic studies, which can be enlightening in the diagnosis of a neoplastic disease and thus contribute to its diligent and efficient treatment. 7,8,15The IS proposed in this work contemplates all the endoscopic and imaging evidence usually observed in SPs as clinical parameters. The score generated in its correlation makes it possible to establish the levels of diagnostic presumption of these neoplasms.
Correlative analysis office-based nasal endoscopy, CT, MRI and biopsies are vitally important in the evaluation of this SPs and they are in conjunction represents an easy and proper guidance strategy for its timely diagnosis and subsequent preoperative planning treatment and follow-up.
Unfortunately review of pertinent literature regarding correlative analysis office-based nasal endoscopy, CT, MRI and biopsies no conclusive data are available on this point. In this study we investigated a large series of SPs to identify differences of presentation y clinical features, behavior and precision diagnosis. Although the available literature is extensive in the individual study of the radiological, histological and phenotypic of SPs, the available correlational studies have generally been limited an only been to evaluate sinus CT scan confronted with MRI findings or common radiological and histological characteristics in these neoplasms. 7-15,20,21
In this study we investigated a large series of SPs to identify differences of presentation y clinical features, behavior and precision diagnosis. With a cohort size of 103 cases (67% IPs, 30% FPs and 3% OPs), this series is one representative study that addressing correlative analysis office-based nasal endoscopy, CT, MRI of sinonasal papillomas. SPs is usually diagnosed in the late stages in average, 1 - 4 years after the first appearance of sinonasal symptoms, therefore, early diagnosis is rare. A simple strategy for early and timely diagnosis office-based that emerges from our study is establishing an index of suspicion of neoplasms.
The establishment of an index of suspicion through a scale proposed in our research based in the correlation of the endoscopic and imaging clinical criteria commonly observed in the patients studied from the initial finding of an endonasal polypoid mass allowed to identify early developed lesions observed in our investigation through this scale was significant, and it reached 19%, in contrast to the incidence reported by some authors, who place it at less than 1%. 22,23 Likewise the establishment of this correlational suspicion scale allowed to identify SPs with moderated (31%), and advanced growth (50%). Statistical validation of precision and diagnostic accuracy observed in the correlation of endoscopic and imaging studies performed as the parameters of the formulated IS revealed a high level of reliability at verifying the suspicion of this type of neoplasms.
Conclusion
Correlational clinical parameters of the proposed suspicion index correlational scale of clinical assessment of SPs and the statistical validation of accuracy diagnostic levels revealed a high level of reliability at verifying the suspicion of this type of neoplasms. Nevertheless, we understand that is important to promote future multicenter studies with large sample studies are needed to verify even more this diagnostic scale.
The present state of knowledge about this neoplasia of unpredictable clinical behavior favors prolonged study.
Conflict of interests
The authors declare that they have no conflict of interest regarding this article.
Data confidentiality
The authors declare that they guaranteed confidentiality in the publication of data of patients.