Introduction
The regional status of lymph nodes stands out as a critical factor influencing prognosis among patients with head and neck squamous cell carcinoma. Indeed, the detection of a single positive lymph node can lead to a decrease in survival rates of up to 50% 1,2.
Understanding the tumor's biological behavior is significantly enhanced by the assigned pathological Tumor Node Metastasis (TNM) stage 3. The pN classification, based on the 8th edition of the TNM Staging System (2017), considers factors such as the evidence of single/multiple metastatic lymph nodes, laterality, size and presence of extracapsular spread (ECS), but overlooks metastatic lymph node burden 4.
The lymph node ratio (LNR), defined as the ratio between metastatic lymph nodes and the total number of nodes dissected, is predominantly influenced by the extent and quality of the neck dissection, along with the scrutiny of histopathologic examination 5.
It is considered a well-established prognostic index in several cancers, particularly in breast, gastric and esophageal cancer 6-8. Several recent studies have proposed the potential use of LNR as a complementary tool to the pathological TNM Staging System in pN+ head and neck squamous cell carcinoma. Nonetheless, its influence on laryngeal cancer remains uncertain 5,9,10.
In this study, we aim to evaluate LNR as a predictive factor for recurrence and cancer-specific mortality in patients with laryngeal cancer with metastatic lymph nodes (pN+) after primary total laryngectomy combined with bilateral neck dissection. Additionally, we intend to determine the optimal cutoff for LNR.
Material and Methods
Study Design:
This retrospective study included patients diagnosed with laryngeal squamous cell carcinoma at the ENT Department of Vila Nova de Gaia/Espinho Hospital, between October/2010 and April/2023. All patients underwent primary treatment for laryngeal cancer through total laryngectomy combined with bilateral neck dissection (level I-V or II-IV), with or without adjuvant radiotherapy/chemoradiotherapy. Excluded from the study were patients who had undergone neoadjuvant treatments, prior neck dissection/radiotherapy, or exhibited distant metastases.
Study Variables:
Demographic, clinical and histopathological variables were gathered from the patients' medical records. The analysis of pathological Tumor Node Metastasis (pTNM) stage was conducted in accordance with the 8th Edition of the American Joint Committee on Cancer (AJCC) Staging of Head and Neck Cancer.
The histopathological grading of squamous cell carcinoma was categorized as follows: G1 - Well differentiated; G2 - Moderately differentiated; G3 - Poorly differentiated. The Lymph Node Ratio (LNR) was calculated as the ratio of positive lymph nodes divided by the total number of lymph nodes dissected, as confirmed through anatomopathological examination.
Statistical analysis:
We conducted a descriptive analysis, using the mean and standard deviation (SD) to summarize continuous variables exhibiting a normal distribution, and the median and interquartile range (IQR) for those displaying a non-normal distribution. Categorical variables were characterized by presenting the number of cases (n) and corresponding percentages. ROC curve analysis was used to ascertain the best cut-off value for risk stratification of LNR. Survival analyses applied the Kaplan-Meier method, and curve comparisons were conducted using the log-rank test. Statistical analysis was performed using SPSS 25.0 software, setting the threshold of statistical significance at p < 0.05.
Results
Patient characteristics
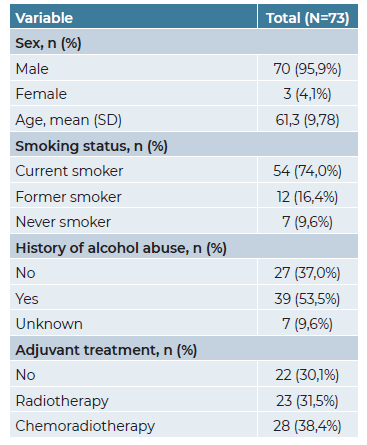
A total of 73 patients - 70 men (95.9%) and 3 women (4.1%) were included in this study, with a mean age at diagnosis of 61.3 years (±SD 9.78).
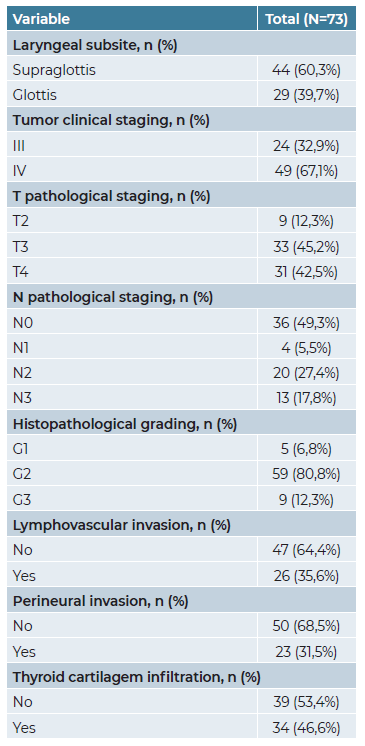
The demographic and clinicopathological characteristics of our cohort are presented in Tables 1 and 2. Regarding tumor location, the majority of patients (60.3%) were diagnosed with supraglottic cancer, whereas 39.7% presented with glottic cancer. Most patients (67.1%) were in stage IV, while 24 patients (32.9%) were in stage III. All patients were submitted to total laryngectomy combined with bilateral neck dissection (level I-V or II-IV). Surgery as a single treatment modality was performed in 22 patients (30.1%), whereas the remaining patients underwent surgery followed by adjuvant radiotherapy (n=23; 31.5%) or chemoradiotherapy (n=28; 38.4%).
Histopathological study
Post-surgical histopathological analysis revealed that the majority of patients had a moderately differentiated (G2) tumor (n=59; 80.8%) and clear resection margins (R0) were achieved in 86.5% of the total cohort. Lymphovascular invasion (LVI) and perineural invasion (PNI) were observed in 26 (35.6%) and 23 (31.5%) patients, respectively. Infiltration through thyroid cartilage was present in 34 (46.6%) patients.
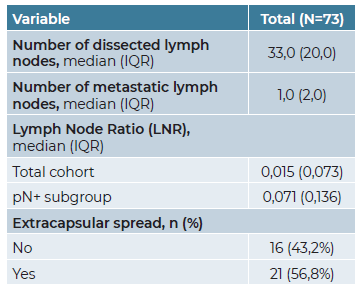
Table 3 illustrates the pathological characteristics of dissected lymph nodes. The median number of dissected lymph nodes was 33.0 (IQR 20.0) and nearly half of the patients (n=36; 49.3%) had a pathologically negative neck dissection (pN0).
Within the entire cohort, the median LNR was 0.015 (IQR 0.073). Conversely, among patients with metastatic lymph nodes (pN+) - comprising 37 individuals (50.7%) - the median LNR was 0.071 (IQR 0.136). In the subgroup of patients with pN+, the median count of metastatic lymph nodes was 2.0 (IQR 2.0). Furthermore, extracapsular spread (ECS) was observed in 21 patients (56.1%).
LNR and tumor persistence/recurrence
The overall prevalence of tumor persistence after treatment was 6.8%, and recurrence rate was 23.3%.
Among the pN+ group, 16 patients (43.2%) had persistence or recurrence of the disease. A higher LNR was observed in the persistence/recurrence group (median LNR=0.159; IQR=0.128) compared to the persistence/recurrence-free group (median LNR=0.060; IQR=0.076), p= 0.007.
Through the ROC curve analysis, the cut-off value of the LNR was identified. We found that a LNR superior to 0.063 had a sensivity of 87.5% and a specificity of 52.4% in predicting persistence/recurrence of the disease in patients with lymph node metastasis (AUC=0.762; p=0.007).
Moreover, in the multivariate analysis, adjusted for potential confounders such as age, extracapsular spread, resection margin, and pathological T category, a LNR>0.063 emerged as an independent predictor of disease persistence/recurrence within the pN+ group (adjusted OR=6.573; 95% CI 1.009-42.835; p=0.049).
LNR and cancer-specific death
Among the entire cohort, the median follow-up time was 2.8 years (range: 4 months - 12.9 years). The all-cause mortality rate was 35.6% (26 patients), with 14 patients experiencing cancer-specific death within a median time of 23.5 months (IQR=26.0) after laryngeal cancer diagnosis.
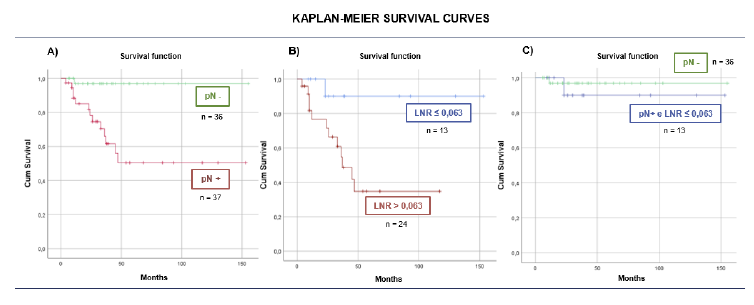
The Kaplan-Meier curve, illustrating cumulative survival, revealed a higher cancer-specific mortality rate among patients with lymph node metastasis (pN+) compared to those without lymph node involvement (pN-), Log Rank test 11.084; p=0.001 (Figure 1-A).
Among patients with lymph node metastasis (pN+), it was observed that survival was significantly longer when the LNR was ≤0.063, Log Rank test 5.016; p=0.025 (Figure 1-B). Remarkably, patients with an LNR superior to 0.063 had over than 8-fold increased odds of cancer-specific mortality in this subgroup (OR=8.640; p=0.008).
However, when comparing the survival curve of patients without lymph node involvement (pN-) and pN+ patients with LNR≤0.063, no significant difference was observed between the groups, Log Rank test 0.439; p=0.507 (Figure 1-C).
Discussion
With advancing knowledge of cancer biology, the traditional TNM staging system is subject to ongoing questioning and revision 4. Although overall survival remains the primary endpoint of TNM staging system 11, incorporating additional endpoints such as disease-specific survival and locoregional recurrence could offer valuable insights for evaluating treatment outcomes 12.
Thus, the objective of this study was to evaluate whether LNR could serve as a complementary tool to the TNM staging system in patients with pN+ laryngeal cancer, in order to predict locoregional failure and disease-specific death.
The incidence of nodal metastasis in laryngeal cancer is estimated to range from 18% to 40%, with a higher proportion linked to tumors located in the supraglottic region 13-15. In our study, 60.3% of patients were diagnosed with supraglottic laryngeal cancer. Overall, 37 out of 73 patients (50.7%) had neck metastasis, which is in line with the percentage described by Lin et al. (45.5%), who analyzed 2094 patients diagnosed with locally advanced laryngeal cancer 16.
Recent studies suggest a worse prognosis linked to elevated LNR values in patients with laryngeal squamous cell carcinoma, although consensus on the exact cut-off remains unsettled 10. Wang et al. stratified 1963 patients with laryngeal cancer and lymph node metastasis into low (LNR ≤ 0.09), medium (LNR 0.09 - 0.20) and high (LNR > 0.20) risk groups and found a 5-year cancer-specific survival of 55.1%, 40.2% and 28.8%, respectively 17. Likewise, according to Künzel et al., a LNR superior to 0.09 was an independent predictor of cancer-specific death (HR=2.065) 5.
In our analysis, it was evident that patients with a LNR greater than 0.063 faced an increased risk of cancer persistence/recurrence (HR=6.573), with cancer-specific mortality being 8 times higher compared to patients with LNR≤0.063. Similarly, Petrarolha et al. reported that a LNR of ≥ 0.060 was associated with decreased overall survival and disease-free survival 18.
Consistent with our findings, Reinisch et al. reported that there was no difference in the survival curves of patients with no lymph node metastasis (pN-) and those with lymph node involvement (pN+) and a LNR lower than 0.060 19.
In fact, tumor-associated lymphangiogenesis promotes lymphatic tumor spread, with excised lymph node count during neck dissection potentially reflecting this process and also tumor biological aggressiveness 20. It is hypothesized that patients with high LNR may experience worse outcomes due to the likelihood of residual positive lymph nodes at the primary site, despite sharing a similar N classification with patients with lower LNR 18.
According to our findings, a LNR superior to 0.063 represents a risk factor for a poorer prognosis in patients with laryngeal cancer with metastatic lymph nodes (pN+). Notably, the adverse impact of a high LNR on disease persistence/recurrence remains significant even after conducting a multivariate analysis to control for confounding factors, namely ECS, pathological T staging or resection margin. Consequently, incorporating LNR calculation into postoperative staging following primary total laryngectomy combined with bilateral neck dissection could serve as a valuable tool for accurately stratifying patients who may benefit from adjuvant therapy 17.
This study has some limitations to consider, particularly the fact that it is a retrospective unicentric study with a small sample. Furthermore, the LNR, as a quantitative measure, is influenced by the extent of neck dissection and the precision of histological analysis. Thus, it serves as an indicator not only of the burden of disease but also of the standards of surgical and pathological quality 5,21. Further prospective larger-scale studies are needed to validate and strengthen our results.
Conclusion
Our results highlight those patients with a LNR>0.063 are likely to present with a more aggressive form of the disease. Therefore, assessing LNR following surgery could be a noteworthy prognostic factor, complementing the TNM staging system in laryngeal cancer with metastatic lymph nodes.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Data Confidentiality
The authors declare having followed the protocols in use at their working center regarding patients’ data publication.