Introduction
Endoscopic mucosal resection (EMR) is the treatment of choice for non-invasive colorectal flat lesions [1]. When en bloc resection is not possible, endoscopic piecemeal mucosal resection (EPMR) may be considered.
Despite its advantages over surgery and submucosal dissection for non-invasive lesion treatment, in both cost and safety [2-4], EMR has a substantial risk of recurrence, particularly when piecemeal resection is performed [5, 6]. A meta-analysis of 33 studies found a recurrent adenoma rate of 20% after EPMR of non-pedunculated colorectal lesions [5]. Therefore, after piecemeal resection, endoscopic surveillance remains a necessity.
Some endoscopic features and procedural variables have been linked to greater risk of recurrent adenoma. Several studies demonstrated the association between large lesion size and adenoma recurrence [6-9]. Additionally, localization in the right colon, namely involving the ileocecal valve or the appendix orifice, and intraprocedural bleeding (IPB) were also associated with recurrent lesions [6, 7]. A previously validated score that defines polyp complexity according to size, morphology, site and access (SMSA score) has been shown to be useful in predicting adenoma recurrence in more complex lesions (i.e., SMSA level 4) [10, 11].
Current guidelines recommend an endoscopic follow-up within 6 months after piecemeal resection of polyps ≥20 mm [12]. Because recurrent lesions frequently do not have advanced histological features and are endoscopically treatable [7, 13, 14], endoscopic surveillance within 6 months for all EPMR has been questioned. A prospective multicenter study by Tate et al. [8] created and validated a tool which aims to predict the risk of recurrent adenoma after EPMR of colorectal lateral spreading lesions: the Sydney EMR Recurrence Tool (SERT), a 0-4 scale that classifies lesions according to size (2 points if ≥40 mm), occurrence of IPB (1 point) and presence of high-grade dysplasia (HGD; 1 point). SERT 0 lesions presented a considerably lower risk of recurrence and could safely undergo first endoscopic surveillance at 18 months, according to the authors.
Our goal is to evaluate the applicability of SERT in predicting adenomatous recurrence after EPMR in a secondary centre cohort.
Materials and Methods
Study Design
A retrospective single centre study was conducted, including consecutive colorectal flat lesions referred for EMR, from March 2010 to February 2018. Lateral spreading lesions with a size of 20 mm or greater, with or without a sessile component, were included if a complete resection was achieved by piecemeal mucosectomy. Exclusion criteria included en bloc resection, previous attempt of resection, evidence of malignancy in the histopathologic analysis and absence of at least 1 endoscopic surveillance.
All patients provided written informed consent for colonoscopy. The study was conducted according to the Declaration of Helsinki and approved by the ethical board of Centro Hospitalar de Leiria, Portugal.
Procedures and Equipment
All colonoscopies were performed with high-definition scopes (Olympus® GIF-H180 or GIF-H185, depending on the colonoscope availability) and with EVIS EXERA III CV-190 Olympus video processors, using room air insufflation. Lesions were resected by EPMR, using oval and/or hexagonal hot polypectomy snares, with fractionated current (Endocut mode Q, effect 3, ERBE VIO 300 S), after submucosal injection of a solution of saline, methylene blue and diluted adrenaline (1:100,000). If residual lesion was suspected at the time of resection, adjunctive endoscopic techniques, including thermal ablation of lateral margins of the mucosectomy site with argon plasma coagulation (APC) and/or cold biopsy forceps excision completion, were used according to the endoscopist’s preferences. Narrow-band imaging (NBI) was used as an adjuvant to white light to evaluate recurrent lesions/scars according to the endoscopist’s preference and expertise.
Data Collection and Outcome Definition
Data were collected by consultation of patients’ clinical, endoscopic and histopathologic records, including age, gender, size of the lesions, morphology, location, access, adjuvant endoscopic techniques, occurrence of bleeding, anatomopathological characterization and endoscopic follow-up. SERT was calculated and each lesion was assigned to the SERT 0 or the SERT 1-4 group. Each lesion was also classified as SMSA level 3 or 4 according to SMSA score.
The primary endpoint was to evaluate the utility of SERT in the prediction of the 6-month adenoma recurrence rate, which was defined by the presence of dysplasia in the anatomopathological evaluation of the recurrent resected tissue. Endoscopic image enhancement techniques like NBI were not used to define recurrent adenoma. Secondary endpoints included the applicability of SERT in other time points, the correlation between SERT and SMSA score and the evaluation of the association between other endoscopic factors and recurrent adenoma.
Statistical Analysis
Statistical analysis was conducted in Statistical Package for the Social Sciences (SPSS), version 23.0 (IBM, Armonk, NY, USA). Mean and standard deviation were used to describe normally distributed variables, while median and interquartile range (IQR) were used to describe skewed distributed variables. Bivariate analysis was performed using Student t test for normally distributed variables, Mann-Whitney U test and Spearman’s correlation coefficient for skewed distribute variables and χ2 test for dichotomous variables. A bivariate logistic regression was conducted for the primary endpoint, considering the following potential confounding variables: age, gender, lesion location (right colon vs. left colon/rectum), lesion accessibility (easy vs. difficult access), use of adjunctive therapy (APC/biopsy forceps) and occurrence of delayed bleeding, defined as haemorrhage after the end of the procedure requiring endoscopic treatment. The Kaplan-Meier method was applied to evaluate time to recurrence in each group of lesions according to SERT classification. A p value <0.05 was regarded as statistically significant.
Results
Study Population
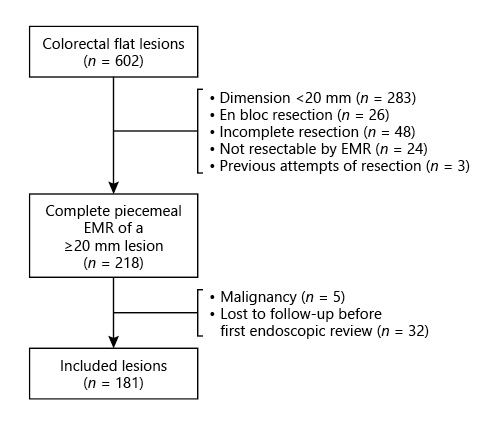
Six hundred and two colorectal flat lesions were considered for inclusion (Fig. 1). Two hundred and eighty-three lesions were excluded for having a dimension inferior to 20 mm and 26 were resected en bloc. In 48 cases, resection was not complete; 24 lesions were not considered resectable by EMR; 3 lesions were excluded due to previous attempts of resection. Five lesions revealed histopathologic features compatible with adenocarcinoma and were thus excluded from the analysis. Additionally, 32 patients were lost to follow-up before endoscopic re-evaluation could be performed and/or had insufficient data in clinical records. Therefore, 181 lesions were included in the analysis, corresponding to 174 patients, with a mean age of 67.8 ± 9.6 years and a predominance of male gender (60.9%; n = 106).
Lesion Characteristics and Procedural Variables
Endoscopic and histopathological features of the included lesions are presented in Table 1. The mean size of the lesions was 33.4 ± 11.5 mm. Morphology was described using the Paris Classification. Most of the lesions were elevated, with or without a sessile component (0-IIa: 39.2% [n = 71]; 0-IIa + Is: 23.2% [n = 42]). The majority of the lesions were located in the right colon (64.1%; n = 116), mostly in the ascending colon (34.3%; n = 62), follow by the rectum (19.9%; n = 36). Access to the lesion was considered difficult in 6.1% (n = 11) of cases.
Table 1 Baseline characteristics of the lesions submitted to piecemeal endoscopic mucosal resection, procedural variables and SERT points

Adjunctive endoscopic therapy was used in 45.3% (n = 82) of cases, namely thermal ablation of lateral margins of the mucosectomy site with APC (37.0%; n = 67) and excision complementation with biopsy forceps (8.3%; n = 15). IPB occurred in 5.0% (n = 9) of cases and delayed bleeding was verified in 7.2% (n = 13). There was 1 case of colonic perforation, treated with clip closure and antibiotic therapy. All complications were successfully managed with endoscopic treatment. Most lesions were tubulovillous adenomas (70.8%; n = 128), followed by tubular adenomas (19.9%; n = 36). HGD was documented in 24.3% (n = 44) of cases.
The majority of lesions was classified as SERT 0 (66.3%; n = 120), followed by SERT 1 (19.3%; n = 35), SERT 2 (6.6%; n = 12), SERT 3 (5.5%; n = 10) and finally SERT 4 (2.2%; n = 4). Sixty-six lesions (36.5%) were classified as SMSA level 4, and the remaining were SMSA level 3.
Endoscopic Surveillance and Residual Lesion Resection
The median time to first endoscopic surveillance was 6 months (IQR: 4-7 months). Visible recurrent lesions were seen in 28.2% of cases (n = 51), with a dimension >10 mm in 4.4% (n = 7). Regular scars were observed in 45.9% (n = 83) of first endoscopic re-evaluations. No scar nor apparent residual lesion was seen in 26.0% of cases (n = 47).
Visible residual lesions were preferably resected by en bloc polypectomy snare resection (36%; n = 18). When that could not be achieved, excision was carried out through EPMR (6%; n = 3) and/or completed with biopsy forceps (64%; n = 32). After resection of the residual tissue, adjunctive ablation of the scar with APC was performed in 13 cases (26%; Fig. 2). All resected tissue was sent to anatomopathological evaluation: 27 lesions (52.9%) corresponded to recurrent adenoma and 24 (47.1%) were hyperplastic tissue (Fig. 3). Nineteen percent (16/83) of regular scars were biopsied according to the endoscopist’s decision. The median endoscopic follow-up time was 11 months (IQR: 6-20 months).
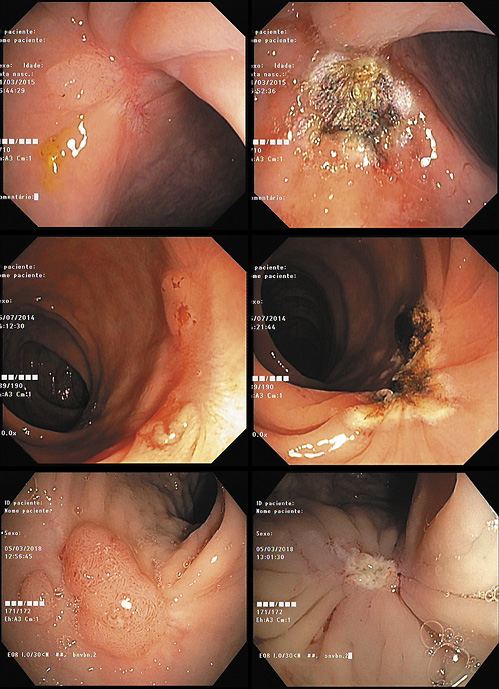
Fig. 2 Endoscopic appearance of recurrent adenoma before (left) and after (right) endoscopic treatment.
Recurrent Adenomatous Lesions
The 6-month adenoma recurrence rate was 16.0% (n = 29). In the vast majority of cases, recurrent adenoma corresponded to visible residual lesions in endoscopic surveillance (93.1%; n = 27/29). However, 2 of the regular scars’ biopsies revealed adenomatous transformation with low-grade dysplasia.
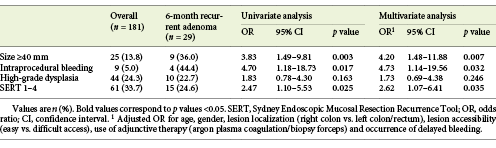
Six-month recurrent adenoma occurred in 11.7% (14/120) of SERT 0 lesions, 17.1% (6/35) of SERT 1 lesions, 25.0% (3/12) of SERT 2 lesions, 30.0% (3/10) of SERT 3 lesions and 75.0% (3/4) of SERT 4 lesions. Two of the three SERT variables (size ≥40 mm and IPB) were associated with recurrence at 6 months (p = 0.003 and p = 0.017, respectively; Table 2). HGD was not associated with recurrent adenoma. Lesions without any of the SERT variables (SERT 0) showed an inferior risk of recurrence (p = 0.016), corresponding to a negative predictive value of 88.3% (n = 106/120) for recurrent adenoma at 6 months. None of the other tested variables (age, gender, localization of the lesion, morphology according to Paris Classification, lesion accessibility, use of adjunctive therapy with APC/biopsy forceps and occurrence of delayed bleeding) showed an association with 6-month recurrence. Nevertheless, the number of SMSA score points correlated with SERT (p < 0.001), and SMSA level 4 was associated with 6-month adenoma recurrence as well (25.8% [n = 17/66] in SMSA level 4 vs. 10.4% [n = 12/115] in SMSA level 3; p = 0.007).
A bivariate logistic regression was conducted to control for potential confounding variables. When adjusted for age, gender, lesion localization, accessibility, endoscopic adjunctive therapy and occurrence of delayed bleeding, the association between SERT and 6-month recurrence remained statistically significant (p = 0.035), with an adjusted odds ratio of 2.62 for SERT 1-4 lesions (Table 2). Additionally, SERT and SMSA score were merged in the same bivariate analysis. Lesions with both high-risk features (i.e., SERT 1-4 and SMSA level 4) presented a 33.3% (n = 11/33) risk of 6-month recurrence (p = 0.003), whereas SERT 0 lesions with SMSA level 3 showed a 9.2% (n = 8/87) recurrence rate at 6 months.
The 24-month recurrence rate was 22.7% (n = 41). At this time point, SERT 0 lesions maintained their inverse association with recurrent adenoma (p = 0.020), with a recurrence rate of 17.5% (n = 21/120) versus 32.8% (n = 20/61) in SERT 1-4 lesions. SMSA level 4 was also associated with 24-month recurrence, with a recurrence proportion of 33.3% (n = 22/66) versus 16.5% (n = 19/115) in SMSA level 3 (p = 0.009). Rectal lesions presented a higher rate of recurrent adenoma at this time point (36.1% [n = 13/36], p = 0.031). However, this difference decreases if only SERT 0 lesions are considered.
The Kaplan-Meier method was used to generate the cumulative incidence of recurrent adenoma (Fig. 4). The cumulative adenoma recurrence rate was higher in SERT 1-4 lesions than in SERT 0 lesions. According to the log-rank test, this difference was statistically significant (p = 0.006).
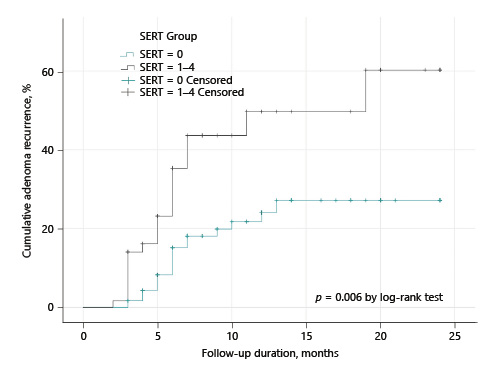
Fig. 4 Kaplan-Meier estimates of the cumulative adenoma recurrence after piecemeal endoscopic mucosal resection according to SERT groups.
Among patients who did not present adenoma recurrence in the 2-year period after EPMR, we were able to follow up 15 patients for 3 or more years. Very late recurrence (more than 3 years after EPMR) was detected in 2 of these patients: one at 3 years and 3 months and the other 5 years after EPMR, both corresponding to initial SERT 1 lesions due to HGD. Both recurrent adenomas displayed low-grade dysplasia. Since no regular scars were biopsied during endoscopic surveillances 3 or more years after EPMR, very late recurrence was only detected in the presence of endoscopic visible recurrent lesions, either with white light or NBI. In the SERT 0 group, the last recurrent adenoma was detected 18 months after EPMR.
All recurrent lesions were resected endoscopically, with the exception of 1 SERT 4 lesion that recurred as a HGD adenoma in 2 consecutive endoscopic re-evaluations. The patient was submitted to right hemicolectomy. In the SERT 0 group, all recurrent adenomas displayed low-grade dysplasia.
Discussion/Conclusion
To the best of our knowledge, this is the first non-tertiary centre study to contemplate the applicability of SERT in adenoma recurrence after EPMR of colorectal lesions. Our results suggest that SERT is useful in predicting adenoma recurrence after EPMR at 6 months and cumulatively during 24 months of follow-up. Two of the three SERT variables (size ≥40 mm and IPB) were independently associated with recurrence, which is in line with previous research [8] and may be explained by a greater complexity of mucosectomy in larger bleeding lesions. However, we could not demonstrate an association between HGD and adenoma recurrence at 6 months. Previous research also failed to demonstrate this association [6, 9]. Arguably, a higher number of patients would be necessary to evaluate the association of HGD with 6-month adenoma recurrence, as was performed by Tate et al. [8]. On the other hand, in our cohort, all recurrent adenomas diagnosed more than 18 months after EPMR corresponded to initial lesions with HGD (and were thus classified as SERT 1-4 lesions), suggesting that HGD may predict late recurrence and that SERT 0 lesions might not need long-term follow-up.
Our findings corroborate two recently published studies [15, 16], which showed similar results in tertiary centres, namely regarding adenoma recurrence rates at 6 months (14% recurrence for flat lesions found by Alexandrino et al. [16] and 20% by Silva et al. [15]). The 6-month adenoma recurrence rates were 11.7% for SERT 0 lesions and 25.0% for SERT 1-4 lesions, which is similar to the rates observed in the study by Tate et al. [8] (10.3 and 27.8%, respectively). For SMSA level 4, we found a recurrence rate of 25.8%, while Alexandrino et al. [16] reported 21.7%.
SERT was correlated with SMSA score and both scores could predict absence of recurrence. Lesions with both low-risk features (i.e., SERT 0 and SMSA level <4) presented the lowest risk of recurrence, with a 90.8% negative predictive value for recurrent adenoma at 6 months. In this group of lesions, all recurrent adenomas displayed low-grade dysplasia and were resected endoscopically. Our results support the suggestion of Tate et al. [8] that the interval to first endoscopic surveillance could be extended in SERT 0 lesions, especially if these lesions also meet the SMSA level <4 criteria.
We found a considerable difference between adenoma recurrence (16.0%) and visible recurrent lesions (28.2%). As seen in Figure 3, endoscopic white light evaluation of residual tissue does not allow the distinction between hyperplastic and adenomatous tissue. This discrepancy has been previously reported [8]. Earlier research has revealed that the absence of visible recurrent lesions has a high negative predictive value for adenoma recurrence, whereas the presence of visible residual tissue has a lower positive predictive value for histologic recurrence [17]. Despite these findings, we detected two recurrent adenomas in endoscopically normal appearing scars. Since the majority of mucosectomy scars was not biopsied when recurrent lesion was not suspected, this number might be underestimated. The fact that NBI was not routinely used to evaluate mucosectomy scars may have contributed to this underestimation.
Our study has some other limitations inherent to a retrospective single-centre study. We excluded 32 patients due to loss of follow-up and/or insufficient data in clinical records. EPMR and surveillance colonoscopies were performed by multiple endoscopists with different experiences in EMR and training in advanced endoscopic imaging. The sample size of our study might have been insufficient to draw some conclusions, namely to associate HGD with adenoma recurrence.
In conclusion, EPMR of large flat colorectal lesions has an important risk of recurrent adenoma, mostly in SERT 1-4 lesions. SERT 0 lesions show a significantly lower rate of recurrence. These findings may allow an extension in the interval to first endoscopic surveillance, if supported by future prospective data.