Introduction
Spontaneous bacterial peritonitis (SBP) is a very common bacterial infection in liver-cirrhotic patients. Its incidence in hospitalized patients varies from 10 to 30% [1, 2]. SBP occurrence is associated with a 30-day-mortality rate of 41% [3]. Early diagnosis and appropriate treatment are essential to improve the prognosis of these patients.
SBP diagnosis is established by a neutrophil count in ascitic fluid ≥250/mm3 in the absence of an intra-abdominal surgically treatable source of infection [2]. Several mechanisms contribute to SBP. The most paradigmatic is bacterial translocation from the intestinal lumen to mesenteric lymph nodes [4]. This concept explains why gram-negative bacteria, particularly Enterobacteriaceae, were once considered the major causative SBP microorganisms, thereby guiding the empirical antimicrobial choice [1, 4, 5].
However, in the last decade a shift in SBP microbial patterns toward an increasing incidence of gram-positive bacteria has been reported [6-11]. Recently, gram-positive bacteria have been reported to account for approximately half of culture-positive SBP [3, 12-18]. Another challenge in SBP treatment is the emergence of multidrug-resistant (MDR) bacteria, especially in nosocomial infections [19-21].
These changes in microbiological profile and bacterial antibiotic resistance raise concern about the efficacy of the current recommended antibiotics [3]. Initial treatment with an effective empirical antibiotic is crucial since resistance to first-line treatment contributes to higher mortality rates [12, 13].
For many years, third-generation cephalosporins (TGC) have been recommended as first-line treatment. Resistance to TGC has become more frequent in recent years, particularly in nosocomial SBP, with resistance rates around 30-40% [12, 15, 16]. Given these resistance rates, empirical treatment with cephalosporins cannot be recommended for noncommunity-acquired SBP [12]. Lutz et al. [12] suggested piperacillin-tazobactam for empirical treatment of healthcare-related SBP and the combination of carbapenem and glycopeptide for nosocomial SBP.
Nevertheless, there is concern about the risk of an increased frequency of more resistant bacteria in hospitalized patients with the use of broad-spectrum antibiotics as first-line treatment [22]. In fact, administration of inappropriate therapy can be associated with increased mortality [23].
Hence, local surveillance of the microbiological scenario and antibiotic resistance must be ensured. In Portugal, there is no recent published information about that issue and, accordingly, we aim to evaluate the microbiological profile and bacterial resistance of SBP pathogens in a Portuguese SBP patient cohort in order to provide adequate tools to select the most appropriate antibiotic treatment.
Methods
Setting and Study Cohort
All consecutive adult (age ≥18 years) cirrhotic patients diagnosed with SBP in which a culture of ascitic fluid was positive were retrospectively evaluated. This study was conducted at the Centro Hospitalar e Universitário de Coimbra (CHUC). Patients were identified using the general diagnostic database of our hospital and searching for all SBP events within the study period (from January 1, 2012, to December 31, 2017).
From patients electronic health records, we collected: age, gender, cirrhosis etiology, liver disease severity (assessed using the Model for End-Stage Liver Disease score and the Child-Pugh score), disease manifestations, first-line antibiotic therapy, duration of hospitalization, details of previous hospital admissions and antibiotics used over the preceding 90 days, history of SBP chemoprophylaxis, baseline laboratory parameters (full blood count, international normalized ratio of prothrombin time, albumin, bilirubin, creatinine, sodium, C-reactive protein), and ascitic fluid parameters (leukocyte and neutrophil counts, biochemical parameters, and culture and in vitro susceptibility tests). Our exclusion criteria were: evidence for secondary intra-abdominal infection, culture-negative specimens, a history of peritoneal dialysis or liver transplant, infection with human immunodeficiency virus or a congenital immune dysfunction, and lack of data on the primary outcome.
Operational Definitions
SBP diagnosis was based on a neutrophil count in ascitic fluid ≥250/mm3. The site of SBP acquisition was classified as nosocomial if the diagnosis was made 48 h or longer after hospitalization and nonnosocomial if the diagnosis was made within the first 48 h of hospital admission. MDR bacteria were defined as those with an acquired resistance to at least 1 agent in 3 or more antimicrobial categories [2].
Complications of cirrhosis such as hepatic encephalopathy and hepatorenal syndrome were defined according to international guidelines [2].
Statistical Analysis
Categorical variables were presented as proportions and continuous variables as means (±SD) if normally distributed or as median (IQR) if they had a nonnormal distribution. The Shapiro-Wilk test was used to assess normality. Univariate comparisons were performed using the χ2 test or the Fisher exact test for qualitative data, and for quantitative data we used the Student t test (if the variables normally distributed) or the Mann-Whitney test (if the variables were nonnormally distributed). Two-tailed p values <0.05 were considered statistically significant. All statistical analyses were carried out using IBM SPSS Statistics software version 22 (IBM, New York, USA).
Results
Patient Characteristics at Baseline
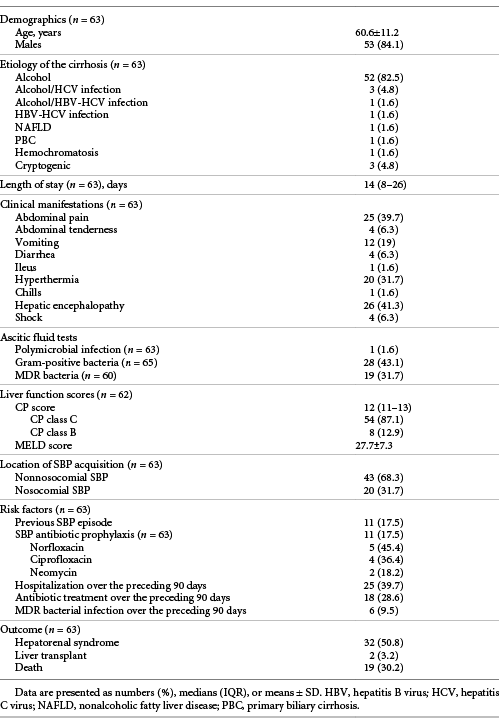
After applying the predefined exclusion criteria, 63 patients with culture-positive SBP fulfilled the eligibility criteria and were considered for the final analysis.
The study cohort comprised 53 men and 10 women. The mean age of the patients was 60.6 years (±11.2). Alcohol was the most frequent etiology of cirrhosis, being present by itself or concomitantly with other etiologies in 88.9% of the patients. Most patients were at an advanced stage of liver disease (87.1%, Child C; 12.9%, Child B) with a median Child-Pugh score of 12 (IQR 11-13). The mean Model for End-Stage Liver Disease score was 27.66 (±7.32).
The median time of hospitalization was 14 days (IQR 8-26, range 1-57 days). The 3 most common presenting symptoms were impairment of the mental state (41.3%), abdominal pain (39.7%), and fever (31.7%).
Eleven patients (17.5%) were receiving SBP antibiotic prophylaxis at the time of admission. Over the preceding 90 days, among the 63 patients, 25 (39.7%) had been hospitalized, 18 (28.6%) had received antibiotic treatment, and 6 (9.5%) had developed an MDR bacterial infection.
All of the baseline characteristics of our sample are displayed in Table 1.
Ascitic Fluid Microbiological Profile
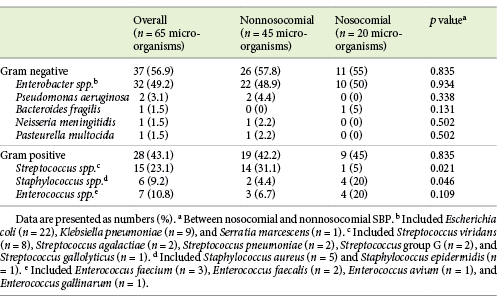
A total of 65 pathogens were isolated in the 63 cases of SBP because 1 patient had 3 different microorganisms isolated in the ascitic fluid (Klebsiella pneumoniae, Pseudomonas aeruginosa, and Enterococcus faecium). Gram-negative bacteria were slightly more frequent than gram-positive bacteria (56.9 vs. 43.1%, respectively). Escherichia coli was the most common isolated pathogen (33.8%), followed by K. pneumoniae (13.8%), Streptococcus viridans (12.3%), Staphylococcus aureus (7.7%), and E. faecium (4.6%).
In a subgroup analysis, we evaluated the microbiological profile between 2 groups of patients, i.e., one group admitted between 2012 and 2014 and the other admitted between 2015 and 2017. The rate of gram-positive bacteria was significantly higher in the first period (58.1 vs. 29.4%; p = 0.02).
The characteristics and the distribution of the bacterial microorganisms are summarized in Table 2.
Antibiotic Resistance among Isolated Microorganisms
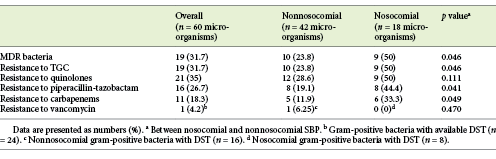
A drug sensitivity test (DST) was available for 60 microorganisms. Nineteen (31.7%) were classified as multidrug-resistant (MDR), including 4 extended-spectrum β-lactamases (ESBL) (producing E. coli; detection rate: 18.2%), 3 ESBL (producing K. pneumonia; detection rate: 33.3%), 1 carbapenemase (producing K. pneumonia [KPC]; detection rate: 11.1%), 2 methicillin-resistant S. aureus (detection rate: 40%), 1 methicillin-resistant S. epidermidis, and 1 VRE (detection rate: 14.3%).
Considering all SBP episodes with available DST, TGC resistance was found in 19 (31.7%) cases, resistance to quinolones was found in 21 (35%) cases, resistance to piperacillin-tazobactam was found in 16 (26.7%) cases, and resistance to carbapenems was found in 11 (18.3%) cases. Considering only gram-positive bacteria with available DST (n = 24), only 1 pathogen (4.2%) showed resistance to vancomycin (E. gallinarum). Our study showed that resistance to quinolones (27.8 vs. 45.8%; p = 0.151), resistance to TGC (25 vs. 41.7%; p = 0.174), and resistance to piperacillin-tazobactam (25 vs. 29.2%; p = 0.721) were not significantly different between gram-negative and gram-positive bacteria. Carbapenem resistance was significantly higher among gram-positive bacteria (41.7 vs. 2.8%; p < 0.05).
When comparing cirrhotic patients admitted between 2012 and 2014 and patients admitted between 2015 and 2017, we identified an equal rate of MDR bacteria (33.3 vs. 33.3%; p = 1.00) between these 2 time periods.
The antibiotic resistance patterns are presented in Table 3.
Impact of Nosocomial Infection on Bacterial Profile and Resistance
Among the 63 patients, 20 (31.7%) had a nosocomial infection. Gram-positive bacteria rates were similar between nosocomial and nonnosocomial SBP episodes (45 vs. 42.2%; p = 0.835). Streptococcus spp. were significantly more common in nonnosocomial SBP (31.1 vs. 5%; p = 0.021), and Staphylococcus spp. occurred more frequently in nosocomial episodes (20 vs. 4.4%; p = 0.046). There were no significant differences in relation to other SBP microorganisms between groups (Table 2).
Considering SBP episodes with available DST, MDR bacteria were more frequent in the nosocomial group compared to the nonnosocomial group (50 vs. 23.8%; p = 0.046). Resistance to TGC (50 vs. 23.8%; p = 0.046), quinolones (50 vs. 28.6%; p = 0.111), piperacillin-tazobactam (44.4 vs. 19.1%; p = 0.041), and carbapenems (33.3 vs. 11.9%; p = 0.049) was more frequent in nosocomial SBP episodes than in nonnosocomial ones, with statistically significant differences between groups in all antimicrobial categories except for quinolones (Table 3).
Impact of Clinical Baseline Factors on the Bacterial Profile and Resistance
Eleven SBP episodes occurred while patients were on SBP prophylaxis. Among them, only 9 had available DST. MDR bacteria were more frequently isolated in patients on SBP prophylaxis than in patients who were not on prophylaxis (55.5 vs. 26.5%; p = 0.119).
Resistance to quinolones was more common in patients on antibiotic prophylaxis with this antimicrobial category than in patients on prophylaxis with neomycin or without prophylaxis (57.1 vs. 31.4%; p = 0.219).
MDR bacteria were significantly more common in patients with history of antibiotic treatment over the preceding 90 days (52.9 vs. 22%; p = 0.02).
Patients with a history of hospitalization or MDR bacterial infection over the preceding 90 days had a higher probability of infection with MDR bacteria, although without statistical significance (44 vs. 21.2%, p = 0.063, and 66.7 vs. 26.9%, p = 0.068, respectively).
Resistance to Empirical Antibiotic Treatment
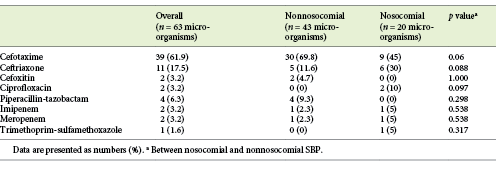
Among all 63 patients, cefotaxime was the most prescribed antibiotic (61.9%), followed by ceftriaxone (17.5%) and piperacillin-tazobactam (6.3%) (Table 4). There were no statistically significant differences between nosocomial and nonnosocomial SBP patients regarding the antibiotic regimen prescribed.
Among the 58 patients with available DST, 17 patients (29.3%) showed resistance to the first-line antibiotic. Resistance to cefotaxime occurred in 12.1% of these patients, resistance to cefoxitin occurred in 1.7%, resistance to ceftriaxone occurred in 6.9%, resistance to meropenem occurred in 1.7%, resistance to piperacillin-tazobactam occurred in 1.7%, and resistance to trimethoprim-sulfamethoxazole occurred in 1.7%. Resistance to first-line antibiotic treatment was more frequent in the nosocomial group than in the nonnosocomial group (44.4 vs. 22.5%; p = 0.089).
Only 22 of the 63 patients underwent a paracentesis after 48 h of treatment to confirm the reduction of the neutrophil count. Among those patients, 14 (63.6%) had concordant laboratory and microbiological responses, i.e., the neutrophil count was reduced to at least 25% of the pretreatment value and the empirical antibiotic covered the microorganism identified on ascitic culture. Half of the remaining 8 patients presented a neutrophil count reduction of more than 25%, but the DST revealed a resistant microorganism which implied the need to switch the empirical antibiotic; the other half of patients had a neutrophil count reduction of less than 25%, but the DST identified a microorganism not resistant to the empirical antibiotic, which did not imply the need to change the first-line empirical antibiotic therapy.
Outcomes
Overall, hepatorenal syndrome occurred in 50.8% of the patients. Hepatorenal syndrome rates was similar between patients with nosocomial SBP and those with nonnosocomial SBP (50 vs. 51.2%; p = 0.932). Comparing patients with MDR bacteria and those with non-MDR bacteria, hepatorenal syndrome was more frequent in the former group but without a statistically significant difference between them (61.1 vs. 47.5%; p = 0.337). Considering patients with resistance to the first-line antibiotic treatment, hepatorenal syndrome occurred in a proportion similar to that of patients who responded to the first-line antibiotic (52.9 vs. 51.2%; p = 0.905).
Only 2 out of 63 patients (3.2%) received a liver transplant during their hospital stay. There was no significant association between nosocomial infection (5 vs. 2.3%; p = 0.538), infection with MDR bacteria or resistance to first-line antibiotic treatment, and the need for transplantation (p = ns for all comparisons).
Nineteen of the 63 patients (30.2%) died during hospital stay. The mortality rate was higher in the nosocomial group (45 vs. 23.3%; p = 0.08) as well as in patients with MDR infection (38.9 vs. 25%; p = 0.282). The mortality rate was similar between patients with resistance to the first-line antibiotic treatment and those without resistance to the first prescribed antibiotic (29.4 vs. 29.3%; p = 1.00).
Discussion
In the present study, we evaluated the epidemiology of causative microorganisms and antibiotic resistance patterns in a cohort of cirrhotic patients presenting with culture-positive SBP in a tertiary care university hospital.
The majority of our patients were men (84.1%) and the main etiology of the liver disease was alcohol (88.9%), as expected from a cohort of cirrhotic patients in Western Europe [3, 12, 21]. In line with previous studies [12, 13, 24], most of our patients presented with advanced liver disease (87.1%, Child C).
Spontaneous ascitic fluid infection is usually associated with a monomicrobial bacterial growth. In the case of multiple organisms on ascitic culture, a secondary bacterial peritonitis should be suspected [2]. In our SBP cohort, only 1 patient had a polymicrobial infection (1.6% of all cases), and any intra-abdominal surgically treatable source of infection was detected. This result is consistent with the findings of other studies. Lutz et al. [12] and Al-Ghamdi et al. [13] described a polymicrobial SBP episode in 2.2 and 1.5% of the patients, respectively.
In our cohort, we identified a considerable proportion (43.1%) of gram-positive bacteria in ascitic fluid cultures, similar to that reported in previous studies [3]. However, our study showed that E. coli still represents the most common microorganism in culture-positive SBP episodes (33.8%). This result was also reported in many other recent studies [3, 12-17, 24, 25], with the prevalence of E. coli among the isolated bacteria in ascitic fluid cultures varying between 22.9 and 40.7%.
Besides the increasing proportion of gram-positive bacteria, another concern is the emergence of MDR bacteria. MDR pathogens have been associated with a high 30-day-mortality rate [25]. In our cohort, 31.7% of the isolated bacteria were classified as MDR bacteria. In a recent retrospective study [21], the authors compared 2 groups of cirrhotic patients with a positive ascitic fluid culture, with one group admitted between 2007 and 2014 and the other between 2015 and 2017. They reported that the proportion of patients with MDR bacteria increased significantly from 22.3% in the first period to 40.7% in the last 3 years. This trend in MDR bacteria rate over time was not noticed in our study, in which an equal rate of MDR bacteria (33.3%) was detected between the 2 time periods (2012-2014 vs. 2015-2017). This finding could be explained by the shorter duration of our study.
Methicillin-resistant S. aureus, vancomycin-resistant enterococci (VRE), and MDR gram-negative bacteria have been reported as a cause of SBP [12]. Among the resistant bacteria identified in our patients, 18.2% were ESBL-producing E. coli. In recent studies [3, 13, 14, 24], the ESBL E. coli strains detection rate has varied between 10.5 and 61.5% [14]. K. pneumoniae was the second most common microorganism in our series, and one third of K.pneumoniae strains produced extended-spectrum β-lactamases. Li et al. [24] described a detection rate of ESBL K.pneumoniae of 20.7% and Guo et al. [14] reported that all K.pneumoniae strains were ESBL-positive. In recent literature [17, 25] the concern about K.pneumoniae infection and its antimicrobial resistance has been discussed. In fact, Klebsiella spp. peritonitis was associated with a poorer prognosis and a higher 30-day-mortality HR compared to those infections caused by E. coli [25]. In relation to gram-positive bacteria, S. aureus was identified in 7.7% of SBP episodes and 40% of these strains were methicillin-resistant. This detection rate was higher than that reported by other authors (26.1%) [24]. VRE infections are of increasing concern in many hospitalized patients [26]. Friedrich et al. [16] showed that infections with enterococci were associated with a poor survival compared with nonenterococci infections. Enterococcus spp. were found in 10.8% of ascitic fluid cultures in our cohort, which is within the range reported by other studies [12, 16, 24, 25], i.e., between 2.9 [13] and 28% [9]. The detection rate of VRE strains was significant in our cohort (14.3%), similar to that (13%) described in a previous study [9]. Because of the increasing prevalence of enterococcal SBP and its poor prognosis when treated inappropriately, clinicians should consider empirical therapy with antibiotics that cover enterococcal species for patients with risk factors for these pathogens [9].
Bacterial antibiotic resistance is an important determinant of SBP prognosis. In our cohort, resistance to TGC occurred in 31.7% of the cases, resistance to quinolones occurred in 35% of the cases, and resistance to piperacillin-tazobactam occurred in 26.7% of the cases. These resistance rates are consistent with recent literature [3, 12, 13].
Since DST usually takes several days to become available, diagnostic paracentesis after 48 h of treatment is essential to raising the suspicion of an infection caused by bacteria resistant to empirical antibiotic based on the finding of a neutrophil count that fails to decrease to less than 25% of the pretreatment value [2]. Based on this ascitic fluid parameter, the need for modification of antibiotherapy should be considered. However, this finding must be integrated with other laboratory and clinical data in order to make a final decision about an empirical change of antibiotic.
Regarding TGC, which have been considered as first-line empirical antibiotic therapy for SBP over the years, resistance to this antimicrobial category has become more frequent among SBP pathogens, with resistance rates around 30-40% [12, 15, 16]. Antimicrobial resistance to TGC has been associated with a poor prognosis, with a significant reduced survival probability [16, 25].
A significant finding in our study is that resistance to carbapenems was found in 18.3% of cases, which is higher than values reported in other series. Lutz et al. [12] reported lack of efficacy of carbapenems in 8% of the isolated microorganisms. In our cohort, carbapenem resistance was significantly higher among gram-positive bacteria (41.7 vs. 2.8%; p < 0.05). Al-Ghamdi et al. [13] and Guo et al. [14] did not report carbapenem resistance among gram-negative microorganisms. Resistance to carbapenems can be a life-threatening factor for the prognosis of SBP patients, a with significantly lower 30-day-survival probability [25].
T vancomycin-resistance rate was only 4.2%, which allows us to affirm that vancomycin continues to be a reliable agent for treating gram-positive infections given its low resistance rates, as reported by other studies [14, 25].
Recent studies have reported that nosocomial infections represent between 37% [3] and 65.1% [16] of SBP episodes. In our cohort, nosocomial SBP occurred in 31.7% of the patients. There was no significant difference in Gram category between nosocomial and nonnosocomial SBP episodes (45 vs. 42.2%; p = 0.835), keeping in line with what has been described by other authors [3, 16]. Considering the microbiological etiology of nosocomial and nonnosocomial SBP episodes, there was a significant difference between these 2 groups regarding Streptococcus spp. and Staphylococcus spp. While Streptococcus spp. were significantly more common in nonnosocomial SBP (31.1 vs. 5%; p = 0.021), Staphylococcus spp. occurred more frequently in nosocomial episodes (20 vs. 4.4%; p = 0.046). Friedrich et al. [16] described that Streptococcus spp. occurred significantly more often in nonnosocomial infections (14.3 vs. 2.2%; p = 0.006) and there was a trend toward more Enterococcus spp. in nosocomial infection (16.3 vs. 31.5%; p = 0.053). Li et al. [21] reported that Enterobacteriaceae were more frequent in community-acquired SBP patients (35.7 vs. 23.0%; p = 0.068), while nosocomial SBP patients had more Enterococci (13.6 vs. 24.3%; p = 0.060) and Staphylococci (20.8 vs. 27.0%; p = 0.314). Li YT et al. [24] showed that Acinetobacter baumannii and P. aeruginosa caused a significantly higher number of nosocomial than non-nosocomial infections.
In our series, MDR bacteria were significantly more frequent in the nosocomial group compared to non-nosocomial group (50 vs. 23.8%; p = 0.046), as reported by previous studies [21]. In fact, nosocomial peritoneal infections have been described as a risk factor for antibiotic resistance [12, 27]. In line with this observation, we detected higher prevalence of antibiotic resistance in all antibiotic classes in nosocomial SBP patients. Resistance to TGC class was 50% in nosocomial SBP episodes, which makes it an unsuitable choice for empirical treatment of nosocomial SBP episodes. For non-nosocomial SBP episodes, TGC had a coverage rate of 76.2%, similar to that described in previous studies [16]. Piperacillin/tazobactam and carbapenems showed the highest antimicrobial susceptibility rate in non-nosocomial-acquired SBP episodes, with coverage rates of 80.9 and 88.1%, respectively, similar to that described in previous studies [16]. However, in nosocomial SBP episodes, we showed a considerable resistance rate for these antibiotics (44.4% for piperacillin-tazobactam and 33.3% for carbapenems). These resistance rates were higher than that previously described by Lutz et. al [12]: 30% for piperacillin-tazobactam and 13% for carbapenems. Based on their results, these authors suggested the combination of a carbapenem plus a glycopeptide for empirical treatment of nosocomial SBP.
In our study, 17.5% of the included patients were on SBP prophylaxis, a proportion similar to that previously reported [3]. We showed a trend towards a greater probability of having a MDR bacteria isolated in ascitic fluid culture of the patients on SBP prophylaxis (55.5 vs. 26.5%; p = 0.119). There was no significant association between previous antibiotic prophylaxis and resistance to quinolones (57.1 vs. 31.4%; p = 0.219). Oliveira et al. [3] showed a significant association between previous use of antibiotic prophylaxis and a new SBP event because of bacteria resistant to quinolones (34 vs. 6%; p = 0.001), but not because of MDR bacteria (29 vs. 14%; p = 0.10). Li et al. [21] did not show a significant microbiological difference between patients with or without antibiotic prophylaxis.
In our study, we also showed that history of antibiotic treatment over the preceding 90 days was significantly associated with an SBP event caused by MDR bacteria (p = 0.02).
In relation to the antibiotic treatment prescribed to our cohort patients, TGC (cefotaxime and ceftriaxone) were used in about 80% of the patients. Approximately 20% of those patients had an infection caused by a microorganism resistant to these 2 antibiotics. Friedrich et al. [16] also showed that the majority (84.1%) of culture-positive patients received antibiotic treatment with TGC, but only 58.1% of those patients received an adequate antibiotic therapy specific to the identified pathogen and, more importantly, they found that all of those patients with incorrect empirical antibiotic treatment had received TGC.
Overall, in our cohort, 29.3% of the patients showed resistance to the first-line antibiotic treatment prescribed, mostly in the nosocomial group (44.4 vs. 22.5%; p = 0.089). This finding is probably related to the fact that MDR bacteria were more common in nosocomial group and consequently the probability of treatment failure was expected to be high. According to previous studies, resistance to the first-line antibiotic treatment significantly increased the 30-day-mortality of SBP patients, underlining that knowledge of local antibiotic resistance patterns is crucial to SBP treatment success [12].
In our cohort, there was no significant association between nosocomial infection, MDR bacterial infection, or resistance to first-line antibiotic treatment and the occurrence of hepatorenal syndrome (p = ns for all comparisons). In a retrospective study, Li et al. [21] showed an increasing incidence of MDR bacteria in recent years, accompanied by an increased incidence of hepatorenal syndrome (from 40.8 to 58.0%; p = 0.007). The occurrence of hepatorenal syndrome was independently associated with the 30-day-mortality of cirrhotic patients with a culture-positive SBP [3].
The in-hospital mortality rate of our culture-positive SBP patients was 30.2%. No significant association was noticed between mortality rate and nosocomial infection or MDR bacteria etiology. The mortality rate among our patients was also similar between patients with resistance to the first-line antibiotic treatment and those without resistance to the first prescribed antibiotic. On the contrary, other studies showed that MDR pathogens were associated with a significantly higher in-hospital mortality [21] and 30-day-mortality [25] and that resistance to the first-line treatment contributed independently to a higher 30-day-mortality [12].
The results of our study should be interpreted taking into account some limitations. First, our study is a single-center analysis, which could limit the external validity of the results. However, this limitation is minimized by the fact that our tertiary care hospital is a referral center for liver disease, receiving patients from other hospitals. Second, the retrospective design of this study could imply some missing data and consequently a selection bias. Third, a limitation of our study is that antibiotic resistance could only be analyzed in patients with culture-positive SBP, and ascitic fluid cultures were positive only in half, or fewer, of the cases [12, 13]. Future studies are necessary to better understand all details of epidemiology and bacterial resistance in our SBP cases, allowing a more efficacious antibiotic therapy.
Conclusion
In conclusion, we described a microbiological profile and antibiotic resistance patterns in a recent cohort of cirrhotic patients, similar to the current European experience. Although the majority of pathogens causative of SBP remain gram negative, our results accentuate a shift to more frequent gram-positive organisms which represent almost half of the isolated microorganisms. New SBP treatment protocols should follow this change. Our data also suggest that TGC or piperacillin-tazobactam might be considered for nonnosocomial SBP, and a carbapenem in combination with a substance targeting gram-positive bacteria might be a better choice for empirical treatment of nosocomial SBP.