Introduction
Rectal neuroendocrine tumors (r-NETs) are rare tumors derived from the neuroendocrine cell system, mainly L-cells and are characterized by the production of glucagon-like peptide, pancreatic polypeptide, and peptide YY. r-NETs represent 27% of all gastrointestinal NETs and have an annual age adjusted incidence of 0.86/100,000 in the USA [1]. The incidence of r-NETs has increased over the past decades due to a heightened awareness of the disease process in conjunction with an enhancement in colorectal cancer screening and improved endoscopic diagnosis [2-4].
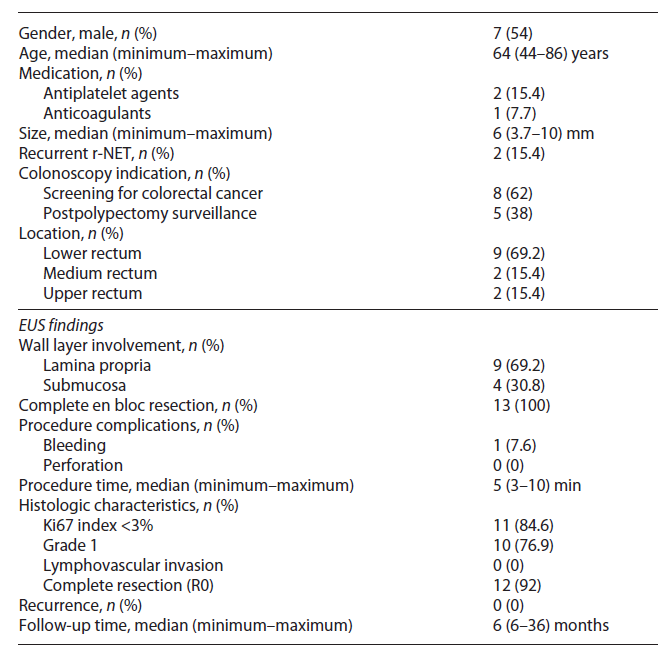
Clinically, most patients are asymptomatic, and the diagnosis is made during screening colonoscopy. On endoscopy, r-NETs are generally small, smooth, round, mobile, yellowish submucosal lesions with a reddish tinge, significant microvessel density, sometimes with a central punctum and found between 5 and 10 cm from the anal verge in 87% of the cases (Fig. 1) [1, 5].
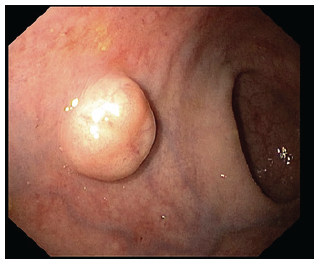
Fig. 1 Endoscopic typical appearance of a rectal neuroendocrine tumor (r-NET): a small, smooth, round, mobile, yellowish, sub-epithelial lesion.
The presence of atypical findings (central ulceration, flattening, or depression) seems to predict a more aggressive form of disease [1]. Biopsy should be taken for histological confirmation in suspected r-NETs over 5 mm and/or high-risk stigmata. Endoscopic mucosal resection (EMR) may be performed in small lesions at index colonoscopy, given the lesser risk of invasion and metastases. Also, a full colonoscopy is required at some point, as part of staging, and to exclude synchronous carcinoma. Endoscopic ultrasound (EUS) is recommended in all lesions with diameter superior to 5-10 mm or with atypical features to assess tumor size, depth of invasion, and the presence of lymph node metastasis (LNM). These r-NETs appear as well-demarcated, homogenous, isoechoic or hypoechoic lesions arising from superficial layers (Fig. 2) [5].
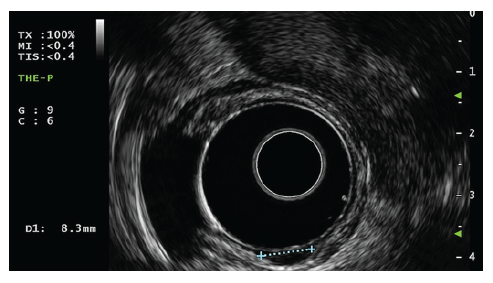
Fig. 2 EUS of a rectal neuroendocrine tumor (r-NET): round, well-demarcated, hypoechogenic nodule with a diameter of 8.3 mm arising from the submucosal layer.
EUS accuracy in determining depth of invasion was reported to be between 92.5 and 100% [2]. In patients with lesions with diameter superior to 10 mm and/or when LNM are detected, additional imaging includes a thoracic, abdominal, and pelvic computed tomography scan to assess for distant metastasis. Magnetic resonance imaging of the pelvis is also indicated for r-NETs with size superior to 20 mm, muscularis propria invasion or beyond, LNM or after an incomplete resection. For well-differentiated r-NETs with diameter superior to 20 mm, muscularis propria invasion or LNM, somatostatin receptor positron emission tomography is useful for detecting metastatic lesions. Fluorodeoxyglucose-positron emission tomography is preferable in poorly differentiated r-NETs. Minimum laboratory studies include serum chromogranin A determination [1].
r-NET management depends on size, grade, and staging. Most r-NETs are smaller than 15 mm and do not invade the muscle layer nor have LNM. Considering these characteristics, most r-NETs can be endoscopically treated and cured.
Conventional EMR is safe and fast but often incomplete, as tumors arise from deeper layers than mucosa. Histological complete resection after conventional EMR is only 72-74% [6, 7]. Endoscopic submucosal dissection (ESD) allows for high rates of complete en bloc resection (90-100%) and excellent diagnostic yield; however, it is associated with higher complication rates and longer procedure times [8].
Device-assisted EMR, namely, EMR using a band-ligation device (EMR-B), cap-assisted (EMR-C) or EMR u-ing a dual-channel endoscope can remove the deeper part of the submucosal layer. Compared with ESD, these techniques resulted in comparable or slightly lower histologically complete resection rate but with a quicker resection time and fewer side effects [9-12]. Recent techniques such as clip-assisted endoscopic fullthickness resection revealed complete resection rates of 95% for r-NETs with 10-20 mm or G2 grading [13].
In conclusion, the optimal strategy for endoscopic resection in r-NETs still requires additional studies to provide strong evidence. Therefore, we aimed to evaluate our experience with the feasibility, efficacy, and safety of EMR-C for r-NETs.
Materials and Methods
Study Design
This was a single-center, prospective cohort study performed from January 2017 to September 2021.
Inclusion and Exclusion Criteria
Patients aged 18 years old or older with histologically confirmed r-NETs up to 10 mm of diameter, without muscularis propria invasion, and without lymphovascular invasion established by EUS. All patients were examined by endoscopy and EUS (Olympus GF-UE160 AL5 radial ultrasound endoscope, 5-10 MHz, with balloon) in our center before endoscopic resection. Patients without endoscopic biopsy confirming r-NET diagnosis or EUS evaluation in our center were excluded.
Definitions
An “en bloc” resection was defined as an excision of the tumor in one piece. A complete pathological resection was defined as an “en bloc” resection of the lesion with a tumor-free margins, that is, the distance from the horizontal and vertical margins to the borders of the tumor was superior to 1 mm. Procedure time was defined as time from the submucosal injection to complete removal of the lesion. Intraprocedural bleeding was defined as any bleeding that required endoscopic hemostasis during the procedure, and delayed bleeding was defined as any bleeding from the resection site that required endoscopic hemostasis or transfusion after the endoscopic resection. Perforation was defined according to deep mural injury classification [14]. Recurrence was defined by the presence of a histologically confirmed r-NET at the previous complete resection of r-NET at least 6 months after the initial resection. At the follow-up EUS, a hypoechoic nodule disrupting any wall layer was considered compatible with recurrence.
Technique Description
A high-definition, single-channel gastroscope was used to perform EMR-C procedures. A mixture of diluted epinephrine (1:100,000) in 0.9% saline solution and methylene blue (1:500,000) was injected submucosally around and beneath the lesion to lift it apart from the muscle layer. A transparent cap for EMR-C was fitted to the scope, and a crescent-type snare was looped along the inner lip of the cap. The lesion was sequentially suctioned into the cap, grasped by the snare, and resected by using the Olympus electrosurgical generator PSD-60 until 2020, with Endocut forced mode 20W effect 2 settings (first 11 cases). From 2021 on, the Olympus electrosurgical generator ESG-300 was used, with Pulsedcut mode 60W effect 4 settings (last 2 cases) (Fig. 3a-f) [10].
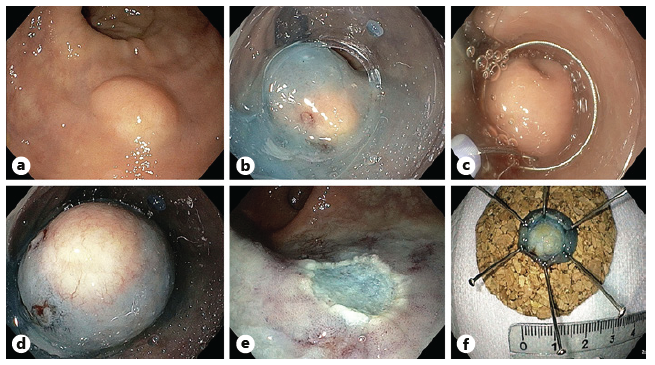
Fig. 3 a Small r-NET. b Submucosal injection with a mixture of diluted epinephrine (1:100,000) in 0.9% saline solution and methylene blue (1:500,000). c Crescent snare suction on the adjacent rectal wall and fitted along the inner rim of the transparent cap. d The r-NET snared with a snare-fitted cap while suctioning it. e The postresection defect. f The resected specimen fixed and measured.
Follow-Up
All patients submitted to complete en bloc resection of r-NETs were followed with standard endoscopy and EUS at 6 and 12 months and yearly thereafter. Biopsy of post-EMR-C scar was done only if recurrence was suspected.
Demographic, Clinical, Endoscopic, and Histologic Variables
Patients’ characteristics: age, gender, antiplatelet and antico-agulant therapy were retrieved from electronic reports. Endoscopic data: tumor size, location, procedure time, macroscopic complete resection, and adverse events were collected from endoscopy report. Ultrasonographic data: tumor size, wall layers involved, and the presence of LNM were collected from EUS report. Histologic data: histopathologic type, Ki67 index, horizontal and vertical resection margins, and lymphovascular involvement were re-trieved from the pathology report. In addition, the World Health Organization classification of tumors of the digestive system was used for histopathological evaluation.
Data and Statistical Analysis
Continuous variables were reported as mean and standard deviation or median and interquartile range, if they have a normal or skewed distribution, respectively; categorical variables as absolute and relative frequencies. The correlation between continuous variables with skewed distribution was evaluated by calculating Spearman correlation. Diagnostic accuracy was evaluated by using the Wilcoxon signed-rank test. Statistical analysis was performed using SPSS version 25 (SPSS Inc., Chicago, IL, USA).
Results
A total of 13 patients were included, 54% (n = 7) were male and median age was 64 (54-76) years. Antithrombotic agents’ intake was reported in 23% patients (n = 3). All patients were asymptomatic. The indications for performing the diagnostic colonoscopy were screening for colorectal cancer (62%, n = 8) and postpolypectomy surveillance (38%, n = 5). Median lesion size on histology, endoscopy, and EUS was 6 (4.5-7.5) mm, 6 (5-7) mm, and 6 (5-7) mm, respectively. There was a strong correlation between size estimated by EUS and histology (r = 0.83, p < 0.01), and by endoscopy and histology (r = 0.88, p < 0.01). EUS accuracy for the depth of invasion was 84.6%. Nine (69.2%) r-NETs were in the lower, 2 (15.4%) in the medium, and 2 (15.4%) in the upper rectum. Overall, 2 (15.4%) were recurrent r-NETs and had been treated previously by conventional EMR. Submucosal involvement was documented in 4 (30.8%) patients. All the tumors were removed en bloc. The median procedure time was 5 (4-8) minutes. Only 1 case of intraprocedural bleeding was reported and was successfully controlled endoscopically with clips. There was no delayed bleeding or perforation. According to histopathologic evaluation, 10 (76.9%) tumors were grade 1, and Ki 67 index was inferior to 3% in 11 (84.6%). No lymphovascular (L0, V0) infiltration was observed in any of the tumors. The histologic complete resection was obtained in 12 (92%). The patient with an r-NET incompletely resected (positive vertical margin) is under endoscopic and EUS follow-up. At 6 months, no evidence of residual or recurrent lesion was found in both exams.
Endoscopic and ultrasonographic follow-up was available in 12 cases (92%). The median follow-up time was 6 (12-24) months. No evidence of residual or recurrent lesion on endoscopic and EUS evaluation was found. There was no distant metastasis on follow-up. This information is summarized in Table 1.
Discussion/Conclusion
Recently, the detection of r-NETs is increasing with the widespread use of screening colonoscopy. Our find-ings are in accordance with this statement because most r-NETs were detected in screening colonoscopy in patients otherwise asymptomatic. As reported in previous studies, and also in our population, most patients were male, and the median age at the diagnosis was 64 (54-76) years [9, 10, 13, 15]. Current guidelines recommend endoscopic resection for r-NETs with diameter lower than 10 mm without risk factors, that is, grade 1, no lymphovascular infiltration nor muscularis propria invasion [1, 16-18]. For higher grade r-NETs (grade 3, Ki67 index superior to 20%), tumors with diameter superior to 20 mm in size or with high-risk factors, surgical resection is recommended. Intermediate grade r-NETs (grade 2, Ki67 3-20%) or lesions with 10-20 mm in size are best managed with surgery. However, if the patient refuses or is less fit for surgery, endoscopy resection, preferably with ESD, can be offered. Figure 4 summarizes the algorithm for treating r-NETs according to current guidelines. EUS was found to be useful for measuring the size and local staging of r-NETs, which is essential for determining appropriate treatment. In our study, tumor size estimation by EUS demonstrated a strong correlation with histologic assessment. Additionally, EUS showed a good accuracy for evaluation of wall layer involvement. Our results are slightly lower than previous studies reporting EUS accuracy in determining depth of invasion of 92.5-100%. Also, for size estimation, Park et al. [19] found a strong correlation between size measurements by histology and EUS (r = 0.91, p < 0.01). In summary, EUS can be applied to facilitate local staging and has been shown to correlate well with depth of invasion and histopathology speci-mens’ size [19, 20].
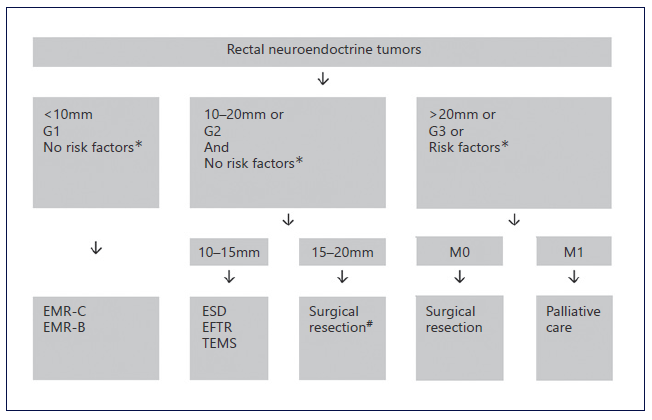
Fig. 4 Summarized management of rectal NETs. *Risk factors: invasion of muscularis propria or lymphovascular infiltration. #ESD, EFTR, or TEMS alternative if no muscular invasion and patient refuses major surgery. EMR, endoscopic mucosal resection; EMR-C, cap-assisted EMR; EMR-B, bandligation device EMR; ESD, endoscopic submucosal dissection; EFTR, endoscopic full-thickness resection; TEMS, transanal endoscopic microsurgery.
The best method for endoscopic resection for r-NETs with diameter lower than 10 mm without risk factors remains controversial. Conventional EMR and polypectomy are fast but often incomplete [1]. Some studies advocate device-assisted EMR or ESD as better endoscopic resection methods.
In our study, including r-NETs with diameter lower than 10 mm without risk factors, EMR-C provided an overall complete resection rate of 92%. Our rate of complete histologic resection is in line with the rate of 94.1% reported in a study conducted by Yang et al. [10]. In our study, EMR-C yielded better results than previously reported for EMR-B (82.8%) [9]. Remarkably, our histologic complete resection rates were similar to those reported for ESD (89.5-94.1%) [8, 10, 21]. The procedure time was 5 (3-10) min. A slightly shorter procedure time was documented by Yang et al. [10] (3.9 ± 1.1 min). Their large experience with EMR-C can explain this difference. Nevertheless, we concluded that EMR-C is a fast procedure, even faster than another device-assisted EMR, such as EMR-B (6.4 ± 3.5 min) [9]. ESD reported times are longer (15-43 min) than those of device-assisted EMR and require a proficient endoscopist in this technique [10, 21]. In our study, only one intraprocedural bleeding, endoscopically treated, was reported, supporting the safety of EMR-C. A study comparing ESD versus EMR-C did not show any differences in adverse events’ rate. A study comparing ESD versus EMR-C did not show differences in the rates of adverse events [10]. There are no complica-tions described from EMR-B procedures for resection of r-NETs [9, 22].
The only patient with r-NET incompletely resected (positive vertical margin) is under endoscopic and EUS follow-up, after multidisciplinary decision. In contrast with colorectal carcinoma endoscopically removed, the true impact of incomplete resection for r-NETs on both recurrence-free survival and overall survival remains unclear. A previous study conducted by Park et al. [19] found residual tumor cells in only 10% of patients considered histologically incomplete but whose resection appeared to be complete endoscopically [23]. Furthermore, true incomplete resection of an r-NET has not yet been proved to be predictive of recurrence or survival [15, 24]. In our study, 2 (15.4%) patients had recurrent r-NETs pretreated with EMR. These recurrent r-NETs were adequately resected by EMR-C. Moreover, no local recurrence was observed during follow-up. Our results underline the efficacy of this technique as salvage treatment as previously demonstrated by Cha et al. [15]. During follow-up time, no evidence of residual or recurrent lesion on endoscopic and EUS evaluation was found, corroborating the favorable natural history of small r-NETs.
According to the European and North American Neuroendocrine Societies, completely resected tumors with a diameter inferior to 10 mm, grades 1 and 2, with no muscularis propria or lymphovascular invasion do not require regular surveillance. However, they postulate that EUS may be required if recurrence is suspected [16]. Unlike r-NETs initial staging, the role of EUS in the follow-up appears to be limited. In a study conducted by Stier et al. [25], EUS appears to have no benefit in the detection of residual r-NET. Until more data are available, we continue to include EUS in surveillance of r-NETs resected endoscopically.
Finally, our study intends to increase endoscopist awareness for the recognition of r-NETs. As reported in previous studies, the overwhelming majority of endoscopists do not suspect the correct diagnosis and perform inadequate endoscopic resection in half of the cases [26].
There are several limitations of this study. First, this was not a randomized control study and is based on the experience of a single tertiary referral center. Therefore, selection bias related to the study design is a major limitation and should be considered before interpreting the results. Second, due to rarity of r-NETs, the patient numbers were small, precluding outcome comparisons within tumor size, location, or grade. Third, the follow-up time was short to assess recurrence as an indicator of therapeutic outcome of r-NETs, which are slowly progressing tumors.
In summary, we demonstrate that EMR-C is a fast, safe, and effective option for r-NETs measuring less than 10 mm without risk factors. Owing to its safety and simplicity, EMR-C might be favored over ESD, and other device-assisted EMR for small r-NETs. However, prospective comparative trials and cost-efficacy studies are needed to better define the role of EMR-C for r-NETs.