Introduction
Pancreatic and peripancreatic collection (PPC) is a common complication of acute and chronic pancreatitis. The incidence of PPC in acute pancreatitis is 5-16%; however, in chronic pancreatitis, it may reach 20-40% [1-4]. The majority of peripancreatic fluid collections complicating acute pancreatitis usually resolve spontaneously within 4-6 weeks of the onset of the attack [5]. Nevertheless, PPC associated with chronic pancreatitis might resolve spontaneously only in a minority of cases [6].
According to duration and amount of necrosis, PPC is categorized into four types: acute peripancreatic fluid collection, pseudocyst, acute necrotic collection, and walled-off necrosis (WON) [7]. The unresolved PPC may be asymptomatic or present with epigastric pain, dyspepsia, fever, gastric outlet obstructive symptoms, or biliary obstruction [8]. Symptomatic and long-term unresolved PPC are the common indications for drainage [9].
Unresolved symptomatic PPC management has evolved dramatically over recent years from surgical or percutaneous drainage into minimal-invasive endoscopic approaches [10]. EUS-guided drainage is the favored approach in current management algorithms, having better outcomes compared to non-guided endoscopic, percutaneous, or surgical drainage approaches [11].
For evaluation of PPC, EUS is the preferred method since it can accurately measure the distance between the GI lumen and the pseudocyst with delineation of a safe nonvascular window for drainage using Doppler US [12, 13]. Likewise, the choice of the stent type used for drainage can be directly influenced by the nature of the fluid content detected by EUS. EUS evaluation of the wall might also affect management decision with the necessity to pre-drainage clarification of diagnosis using EUS-FNA when suspecting cystic neoplasms with focally enlarged/thickened wall [14].
Regarding that EUS-guided transmural drainage needs multiple steps and lots of resources, it would be more wise and efficient if the number of steps is to be minimized, providing shorter time for the procedure as well as using fewer resources while maintaining the efficacy and patient safety of the procedure. A systematic review by Bang et al. [15] concluded that current evidence does not favor routine placement of metal stents over conventional plastic stents for transmural drainage of PPC. Additionally, there is a conflict in current practice about the number and caliber of the plastic stents for EUS-guided drainage of PPC [16]. We tried in this study to alleviate this conflict about the sufficient number of plastic stents needed for pancreatic pseudocyst drainage and evaluate the efficacy of using only one wide-diameter double-pigtail (DP) plastic stent.
Methods
This is a multicenter prospective study for cases of EUS-guided PPC drainage in the endoscopy units of four tertiary centers be-tween January 2017 and February 2020. Patients with symptomatic pancreatic pseudocyst that unresolved for at least 6 weeks after the last episode of pancreatitis were included in this study (Fig. 1). We excluded patients with major comorbidities being unfit for general anesthesia (3 cases), those with multiple cyst septation (7 cases), or >30% necrosis of the cyst content (8 cases) and patients with moderate to marked abdominal free fluid (4 cases). Patients’ medical data were recruited including detailed history for symptoms of pancreatitis (epigastric pain radiating to the back), onset, duration, severity, and possible etiology of pancreatitis (alcoholic, biliary, etc.), basic laboratory investigations, and radiological evaluation including abdominal ultrasound, contrastenhanced CT and or MRCP. Before EUS-guided intervention, cyst fluid analysis (fluid amylase, CEA, and cytological examination) was achieved for confirmation of the diagnosis of PPC.
The procedure time was defined as the elapsed time from the first image of the lesion for the EUS procedure which was obtained to the confirmed image of placement of the pigtail stent into the cyst. Technical success was defined as successful and appropriate placement of the DP stent in the transmural tract. Follow-up examinations, including CT, were performed within 1 month after stent placement to assess complete resolution or a decrease in the sizes of cysts with clinical symptomatic improvement. Treatment success (or clinical success) was defined as a partial (reduction of > 50% of the large axis) to complete resolution of the drained cysts with symptomatic improvement on follow-up CT at 4 weeks.
Endoscopic Procedure and Outcome Assessment
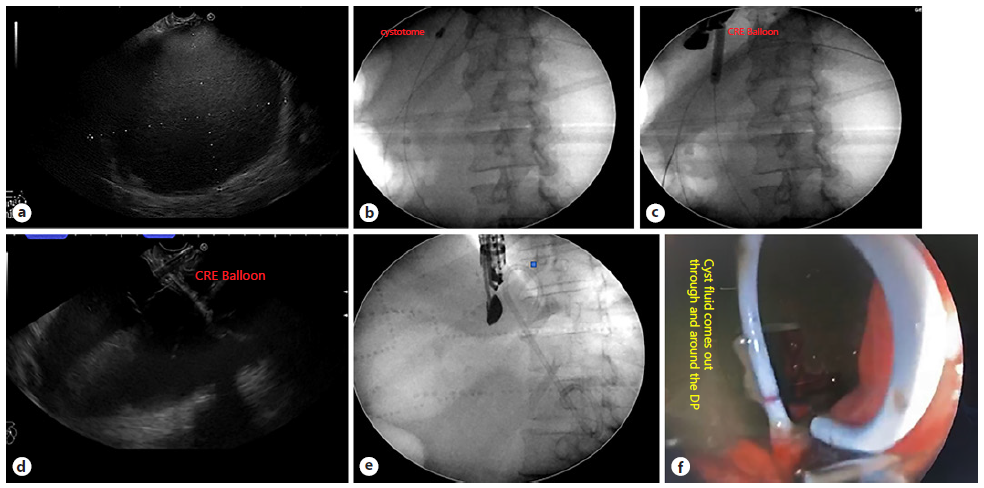
EUS-guided drainage was performed by three experienced endosonographers with long experience in the field of therapeutic EUS using linear-type echoendoscope (Pentax EG-3870UTK “PENTAX Medical, Tokyo, Japan, attached to a Hitachi - Aloka Avius processor “Hitachi, Tokyo, Japan” and Fujifilm EG-580UT “Fujifilm Global, Tokyo, Japan” with SU-1) in all cases with the deployment of one 10 Fr DP plastic stent either through the transgastric or transduodenal approach. Technical success was defined as successful transmural stent placement into the PPC, while clinical success was an improvement of symptoms and resolution of the fluid collection or decrease for more than 50% as determined by contrastenhanced CT performed 1 month after the procedure without recurrence after stent removal within the follow-up period (6 months from the procedure). The detailed technique, duration of the procedure, related complications, and intraoperative complications (bleeding, perforation, leak, stent mal-deployment, or migration) were assessed. Also, early postoperative complications like pain, fever, nausea, vomiting, peritonitis, or pneumoperitoneum and late complication like stent migration, recurrence, pancreatitis were reported. The procedure technique involved EUS characterization of the PPC regarding size, wall thickness, distance from the GI lumen, percent of solid components, exclusion of mural nodules, and choosing the shortest vascular free tract for possible drainage. Subsequently, puncturing of the pancreatic pseudocyst wall was achieved in all cases using a 19-gauge FNA needle under the guidance of the Doppler US, followed by confirmatory aspiration of the content. Afterward, a 0.035-inch guidewire was inserted through the needle into the pseudocyst lumen, forming 2-3 coils under both EUS and fluoroscopy guidance to prevent any potential dislodgment. Furthermore, graded dilatation of the needle tract was achieved using electrocautery (6 or 10 Fr cystotome or needle knife) followed by dilatation using either a 10 Fr Soehendra dilator (Soehendra® Biliary Dilation Catheter) or dilatation balloon of different sizes, 10-11-12 or 12-13.5-15 (Cook® Hercules Dilation Balloon or Boston Scientific® CRE balloon). Finally, under the guid ance of EUS and fluoroscopy, a wide-caliber, 10 Fr DP plastic stent was deployed over the guidewire into the pancreatic pseudocyst with the consequent flow of the content into the GI lumen under an endoscopic view, confirming a successful technique (Fig. 2). All procedures were done under general anesthesia with endotracheal intubation, while the patient is in the left lateral position.
Statistical Analysis
Summary statistics were used to describe the characteristics of the study population (median, ranges for describing quantitative variables, frequency, and percentage for categorical variables). The Mann-Whitney U test was used to compare continuous variables, while χ2 and Fisher’s exact tests were used to compare categorical and dichotomous variables between study subgroups. Logistic regression was used to detect the independent factors affecting clinical outcomes. All statistical analyses were performed using IBM® SPSS software (version 23) for Windows. p value was evaluated as two-tailed, and significance was established at a p < 0.05 level.
Results
Study Population Baseline Characteristics
A total of 57 patients diagnosed with PPC were re-ferred for EUS-guided pseudocyst drainage. Twenty-two patients were excluded (3 patients with major comorbidities, 7 had multiple cyst septation, eight cases had >30% necrosis of the cyst content, and 4 patients with moderate to marked abdominal free fluid). Thirty-five patients were enrolled (19 females/16 males, median age 40 years, range; 3-73), and patient and clinical characteristics are demonstrated in Table 1. While most of the patients were referred directly to EUS drainage, only 4 (11.4%) patients experienced a prior failed trial of percutaneous drainage approach. Idiopathic pancreatitis was the most common etiology (n = 19, 54.3%) followed by biliary (n = 5, 14.3%), post-traumatic (n = 4, 11.4), two post-chemotherapy for acute lymphocytic leukemia, and one case of alcoholic, post-distal pancreatectomy, postsplenectomy, post-ERCP, and primary hyperparathyroidism. All the patients were symptomatic with abdominal pain as the most common symptom (n = 24, 68.6%), early satiety, and dyspepsia in (n = 10, 28.6%) in addition to 1 patient who presented with a picture of gastric outlet obstruction and persistent vomiting.
Endoscopic Details and Outcomes
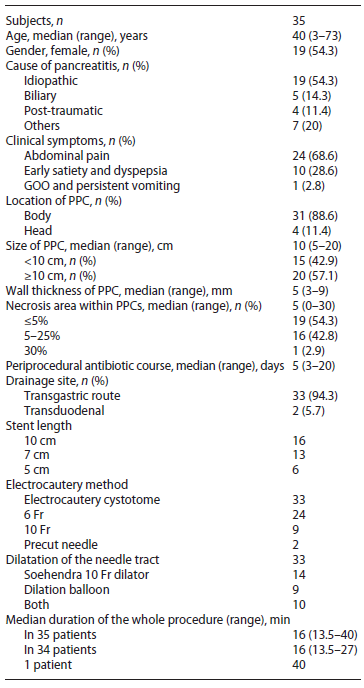
Most of the PPCs were located at the pancreatic body (n = 31, 88.6%). The median size of PPC was 10 cm (range; 5-20) with most of patients >10 cm (n = 20, 57.1%). Median percentage of necrosis within PPCs was 5% (range; 0-30) with 19 patients (54.3%) ≤5%, 16 (42.8%) between 5 and 25% and 1 patient with 30% necrotic area. All patients had a periprocedural antibiotic course of 3rd gen eration cephalosporins or quinolones with a median period of 5 days (range; 3-20). EUS drainage through the transgastric route was the predominant procedure (n = 33, 94.3%). All stents used for the drainage were 10 Fr DP plastic stents with 10, 7, and 5 cm lengths. Electrocautery cystotome (6 or 10 Fr) were used for primary tract dilatation in 33 patients (94.3%) and precut needle in only 2 patients (5.7%). Subsequent dilatation of the needle tract was achieved in 33 patients using a Soehendra dilator 10 Fr alone in (n = 14, 40%), dilation balloon (median 8 mm, range; 4-15) in (n = 9, 25.7%), or subsequent dilatation by both in (n = 10 patients, 28.6%). The median duration of the whole procedure in 34 patients was 16 min (range; 13.5-27), and only one case with mal-deployment of the stent into the lumen of the cyst completed using another stent within 40 min.
Technical success was achieved in all the cases despite few difficulties encountered in 5 patients including difficult access in the duodenal bulb in 2 patients, small-sized stomach in one child case (3 years old), shearing of the wire with the need to repuncture in one case, and a mal-deployed stent into the PPC lumen in 1 patient. Nonetheless, clinical success was encountered in 32 patients (91.4%) with the improvement of patient symptoms and resolution of the cyst without re-accumulation on follow-up. The three failed cases had a relapse of the cyst after an initial good response with the need for re-intervention in 1 patient with percutaneous drainage and repeated EUS-cystogastrostomy in another patient. The third patient of the failure group was misdiagnosed as pancreatic pseudo-cyst due to misleading laboratory data of cyst fluid analysis with re-accumulation after early good symptomatic response. The repeated analysis revealed a diagnosis of mucinous cystic neoplasm and ultimately the patient died with liver metastasis. In addition to the three cases with clinical failure, minor post-procedure adverse events were encountered in 3 patients (8.6%) including post-procedure abdominal pain in 1 patient which improved gradually and fever in 2 patients with resolution after conservative management including antipyretics and broad-spectrum antibiotics. No perforations or bleeding were encountered.
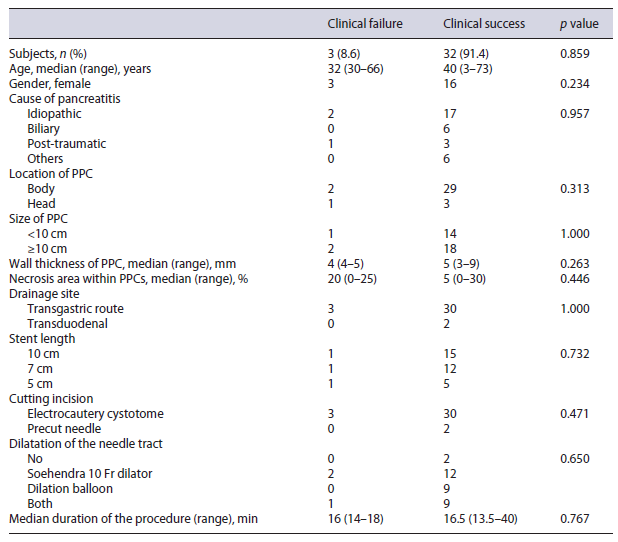
Comparison between clinical success and failure sub-groups revealed no significant difference regarding patient clinical data and PPC characteristics (Table 2). Also, multivariable logistic regression showed no independent factors affecting clinical success (Table 3).
Discussion
This study showed that the management of symptomatic pancreatic pseudocyst by EUS-guided cystogastrostomy with the insertion of a wide-caliber single-pigtail plastic stent is technically feasible, safe, and effective and leads to shorter procedure time. As the general trend in most medical procedures nowadays is evolving toward the easiest, short duration, minimally invasive, fewer resources consuming with the highest efficacy approaches, our study revealed that using one wide-diameter DP plastic stent for symptomatic pseudocyst drainage was safe and effective. In our patients, we had faced only minor adverse events in 3 patients (8.5%) including fever and post-procedure abdominal pain; both have resolved with conservative treatment.
Technical success was achieved in all cases (100%) of our cases with the insertion of a transmural pigtail stent despite the difficulties encountered in few cases (those with transduodenal route) which emphasize the easiness of the technique with decreasing the number of approach steps using only one stent. Clinical success was achieved in 91.4% of our patients with the resolution of patients’ symptoms and no recurrence of a cyst with follow-up after 1 month, 6 months, and 12 months. Our study showed a recurrence rate of 5.7% after an initial good response which needs reintervention (one repeated endoscopic cystogastrostomy and the second underwent percutaneous drainage). This matched with the results of a systemic review that analyzed the mean clinical and technical success of 56 studies (each enrolled more than 10 patients Regarding data using a single-pigtail stent, our results go in line with data reported by retrospective studies showing that drainage of PPC and procedure-related adverse events are not affected by the number or size of used stents and that a single stent may be enough for safe effective drainage [16, 17]. Lin et al. [16] found that clinical success for using a single stent was 93.9% (46/49) versus 97.4% (37/38) for multiple stents (p = 0.799). Secondary infection for single-stent drainage was more than multiple stents (18.4% vs. 5.3%) (p = 0.134). Secondary infection also was more for stent diameter 10 F or more versus 8.5 F or less (17.2% vs. 3.4%; single or more stents) (p = 0.138). Bang et al. [17] also evaluated the number and size of the plastic stent with the treatment outcome in 122 patients, and they found that no relationship between the number or the size of the stent with the treatment success or number of re-intervention (overall success was 94.3%, 83.6% with one intervention and 10.7% with re-intervention and 5.7% failed endoscopic treatment).
Our study has included symptomatic patients with unresolved PPC for more than 6 weeks enabling the cyst wall to be well-defined. The main etiologies for acute pancreatitis and related complication worldwide are gall-stones and alcoholism [18]. Most of our cases had idiopathic or biliary etiologies (68.6%). Alcoholism as a cause of pancreatitis is not so common in the Egyptian population due to the lower incidence of alcoholism in Egyptian society.
The transgastric drainage was regarded as the most common drainage site [19] which is consistent with our study with 94.3% of patients underwent transgastric PPC drainage. Regarding needle tract dilatation, a single-center retrospective study by Kitamura et al. [20] has shown that the use of electrocautery dilator for the needle tract during PPC drainage was shown to be safe and effective. These data have favored the use of cautery dilatation for all cases in our study which in turn has facilitated stent deployment without major related adverse events. Also, the use of electrocautery as the first dilatation device can fasten the procedure. This matched with Kitamura et al. [20] that showed a significant difference in procedure time between the electrocautery group (with mean time 30 ± 12 min) than non-electrocautery group (52 ± 20 min).
All included patients received periprocedural antibi otic course of 3rd generation cephalosporins or quino-lones for a median period of 5 days. While broadspec trum antibiotics are strongly recommended for patients with an infected pseudocyst either empirically or based on culture sensitivity [21], its prophylactic role for non-infected pseudocyst has not been studied [7].
The median duration of the procedure in most cases was 16 min (range; 13.5-27). This short procedure time is mostly related to the insertion of one stent rather than multiple stents. Multiple-stent placement is more difficult, takes a longer time, and has a higher risk of complications.
Regarding hospital stay, the median hospital stay was 3 days (2-7 days). However, in Lin et al.’s [16] work, the mean length of hospital stay was 9.9 ± 10.1 days (range 1-50 days). The short hospital stay in our study is due to exclusion of patients with WON and cyst with more than 30% necrosis, and this indicates the safety and less complication related to the procedure.
Despite our study was prospective and multicenter, we have some limitations including a relatively small number and single-armed protocol. A large number of randomized controlled studies are needed. In conclusion, management of symptomatic pancreatic pseudo-cyst by EUS-guided cystogastrostomy with the insertion of a wide-caliber single-pigtail plastic stent is technically feasible, safe, and effective with short procedure time.
Key Summary
• There is a conflict in current practice about the number and caliber of the plastic stents for EUS-guided drainage of PPC.
• Drainage using multiple plastic stents may be time-consuming with the consumption of more accessories through multiple dedicated steps with increased risk of complications and loss of access during the exchange procedures.
• The use of a single wide-caliber DP stent is technically feasible and effective with high clinical success and reduced procedure steps with less time, accessories, and complications.