Introduction
Nonmalignant portal vein thrombosis (PVT) is a common vascular disorder that develops in advanced liver disease [1]. Patients with cirrhosis have a higher risk of developing hepatocellular carcinoma (HCC), and therefore, it is very important to rule out tumoral invasion in those who develop PVT since this influences therapeutic strategy [1].
The management of PVT starts with an adequate evaluation and risk stratification of the patient at the time of diagnosis. This implies an estimate of the age of the thrombus, whether acute (<6 months) or chronic (>6 months) and the extension and degree of luminal occlusion of the portal vein (PV), splenic and superior mesenteric veins, and exclusion of HCC with cross-sectional imaging with either contrast enhanced computed tomography (CT) or magnetic resonance imaging (MRI) [2].
As the incidence of PVT increases with increasing severity of liver disease, the clinical challenge is to choose the patients who will benefit most from treatment, which in most cases relies on anticoagulation [3]. However, as these are very complex patients often with alcohol dependence, concomitant thrombocytopenia, and comorbidities including chronic kidney disease, management of PVT should be individualized [4].
Epidemiology
PVT is common in patients with cirrhosis but is relatively rare in the general population [1]. In a study involving more than 20,000 autopsied patients, the prevalence of PVT was 1%, with patients with cirrhosis being almost 8 times more prone to develop this condition [5]. The incidence of PVT increases with increasing severity of cirrhosis and is more common in patients belonging to Child-Pugh class B and C and those with high MELD score [6].
In the study by Nery et al. [7], in which 1,243 patients with cirrhosis were prospectively evaluated, the 5-year cumulative incidence of PVT was 10.7%. In a prospective study involving 241 patients, the 1- and 3-year incidence of PVT was 3.7% and 7.6%, respectively [8]. In another prospective study involving 369 patients, the incidence of nontumoral PVT was 1.6%, 6%, and 8.4% at 1, 3, and 5 years, respectively [3]. The prevalence of PVT increases with the severity of the cirrhosis, being less than 1% in patients with compensated cirrhosis and varying between 8% and 25% in those who are candidates for liver transplantation [9].
PVT is partial in 73%-86% of patients and is often asymptomatic [7]. Approximately 40% of PVT will have spontaneous resolution, typically within 3 months, although there are no clearly identified clinical predictors of recanalization [1]. Nonetheless, progression or stability is more common than regression in untreated PVT [10]. The risk of rethrombosis is also significant in these patients and may occur in a third of patients, and therefore, surveillance for de novo PVT should be continued after recanalization [11].
Risk Factors
The pathophysiology of PVT is multifactorial and, like other venous thrombosis, is believed that thrombotic risk is due to blood stasis, hypercoagulability, and endothelial injury, which are the components of Virchow’s triad [1]. Nonetheless, the pathophysiology of PVT seems to be unique, and the relation of these three with it is not totally clear [12].
The splanchnic circulation has unique conditions related to its slow-flow and high-volume hemodynamics that are disturbed by cirrhosis and subsequent portal hypertension (PHT) [1]. The velocity of PV flow is an independent predictor of PVT development, especially in patients with PV blood flow velocity <15 cm/s, making these patients a high-risk subgroup, probably due to blood stasis [6, 13].
Presence of esophageal varices and prolonged pro-thrombin time are independent predictors of development of PVT [7]. Additionally, previous decompensation of cirrhosis and thrombocytopenia has been found to be independent predictors of development of PVT [8].
A study conducted in virus-related cirrhosis suggested that large portosystemic collateral vessels contribute to increased risk of PVT, which could be explained by the steal effect that reduces the PV blood flow [14]. A recent meta-analysis concluded that nonselective β-blockers, used for primary and secondary prophylaxis of variceal bleeding, could increase the risk of PVT in cirrhotic patients [15]. However, some authors defend that the main driver for the higher risk of developing PVT is the severity of PHT, which often correlates with more advanced liver disease and leads to the prescription of nonselective β-blockers, and not the use of the medication itself [3].
Patients with cirrhosis have complex hemostatic alterations with a delicate balance between procoagulant or anticoagulant factors, with a tendency to tip toward thrombosis in the presence of inflammation and infection [3]. The assessment of prothrombin time and international normalized ratio does not truly represent the hemostatic balance of these patients who are not naturally anticoagulated but who actually have a tendency for thrombosis [16]. Although prothrombotic disorders, like congenital or acquired thrombophilias or myeloproliferative neoplasms, are associated with noncirrhotic PVT, the screening for thrombophilic disorders in patients with cirrhosis is currently not yet recommended [2]. A large recent prospective study could not prove a direct link between genetic and acquired prothrombotic factors and the development of PVT in cirrhotic patients [12].
It has been postulated that the direct and indirect damage to portal venous endothelium is mostly promoted by bacterial translocation, inflammation, and endotoxemia in patients with cirrhosis due to the underlying PHT [10]. The association of these conditions with development of PVT needs confirmation with robust studies, but previous decompensation of cirrhosis and thrombocytopenia appear to correlate well with its development, indirectly reflecting the pathophysiological role of the severity of PHT in the development of thrombosis in the splanchnic circulation [8]. Other independent risk factors for the development of PVT in cirrhosis that have been determined include obesity, metabolic syndrome, and nonal-coholic steatohepatitis [17, 18].
The gap between pathophysiology of systemic venous thrombosis and thrombosis in portal vascular territory has been a matter of debate [12]. A recent study in a series of 63 nonmalignant PVT in patients with cirrhosis showed that tunica intima thickening of the vessel wall prevails in a significant proportion of patients rather than fibrin-rich thrombus, which may explain the lower PVT recanalization rates with anticoagulant therapy. This could offer an explanation for the limitations of anticoagulant therapy in some patients. Nonetheless, the study has important limitations and more studies are required to validate this new paradigm in the pathophysiology of PVT [19].
Diagnosis and Classification
In patients with cirrhosis, PVT is often asymptomatic and it is diagnosed incidentally during semestral screening for HCC with abdominal ultrasound with Doppler [20]. Nonetheless, PVT should be suspected in any patient with cirrhosis who develops a clinical decompensating event or abdominal pain [1].
The current guidelines [9, 21] do not provide a formal recommendation to screen these patients for PVT, but as they already undergo abdominal ultrasound screening for HCC every 6 months, the assessment of PV could be done at the same time with abdominal ultrasound ideally with Doppler, which has a higher sensitivity to detect a thrombus in the PV without consuming additional resources and time [11].
Abdominal ultrasound with Doppler is the first-line approach to detect PVT and some imaging features may allow distinction between acute or recent (<6 months) and chronic (>6 months) thrombosis [2]. In an acute PVT, the clot is normally hypoechoic or isoechoic with a mildly dilated PV, in contrast to the hyperechoic material and the cavernous transformation with multiple small vessels in chronic PVT [2]. The performance of abdominal ultrasound with Doppler is not perfect, with a sensitivity of 90% for complete PVT and 50% for partial PVT, with the additional limitation of being operator-dependent [11]. Additionally, there may be technical difficulties in evaluating concomitant superior mesenteric vein (SMV) thrombosis in these patients [10].
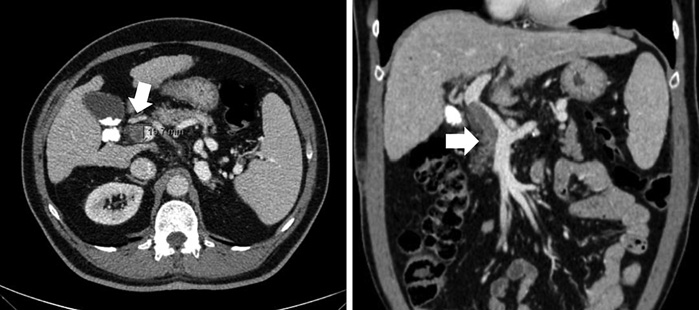
The detection of PVT on abdominal ultrasound with Doppler should be followed by a cross-sectional imaging method, contrast-enhanced abdominal CT, or MRI to confirm the diagnosis, to determine the age of the thrombus and extent and degree of luminal occlusion of the splanchnic circulation by the thrombus, and to rule out HCC and other abdominal malignancies [10]. Anatomically, PVT involves mainly the PV trunk and branches and may involve the SMV and splenic vein [13] (shown in Fig. 1).
The lack of standardized nomenclature in PVT has been a problem, and in order to address this issue, the American Association for the Study of Liver Disease (AASLD) proposed a new standardized nomenclature for the description of PVT [22]. In terms of time course, PVT is classified as recent (<6 months) or chronic (>6 months) and a reference is made to cavernous transformation, which is associated with chronic PVT and lower probability of PVT recanalization [22]. With regard to the severity of occlusion of the main PV, degree of luminal occlusion is classified as <50% and >50%. Finally, during the follow-up, PVT should be defined as progressive, stable, or regressive [22]. All these definitions have important implications on management and outcome.
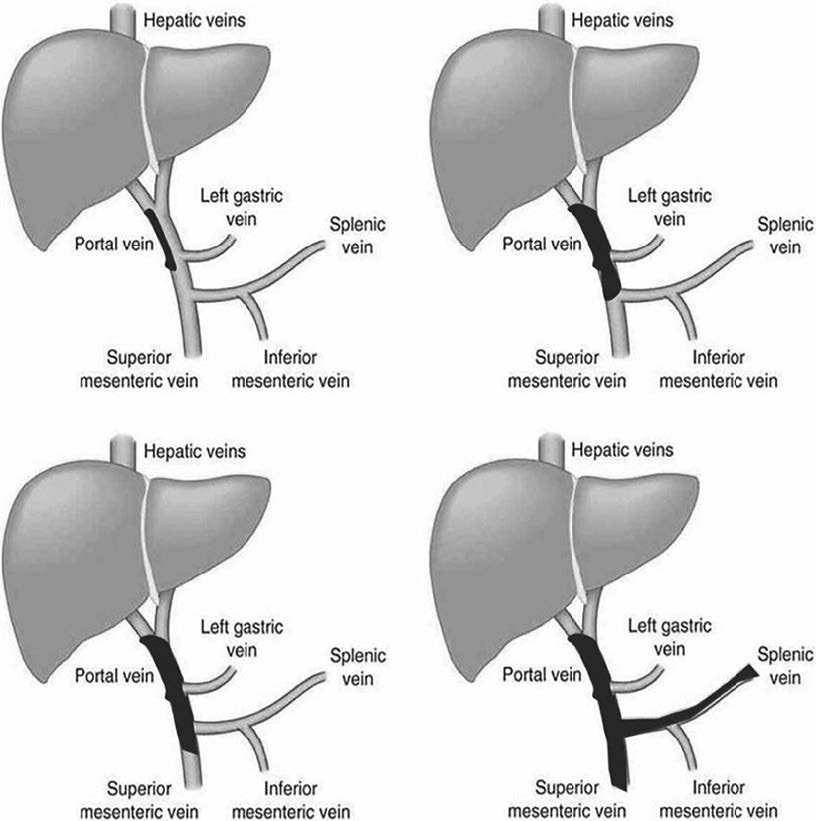
Despite several proposed classification systems, the one suggested by Yerdel et al. [23] using anatomic criteria continues to be widely used, because of its simplicity and reproducibility, which includes: site, degree of occlusion, and extension of the thrombus as shown in Figure 2 [24]. As this classification is only anatomical, although it is useful for surgical management, including liver transplantation, it lacks information for a clinical therapeutic decision [25].
Recently, Sarin et al. [25] proposed a more integrated classification taking into account also functional aspects including the occurrence of symptoms and timing of thrombosis. This classification has been developed for patients with cirrhosis, with the possibility of adding other variables like anticoagulation, type of underlying liver disease, and presence of clinical significant PHT [25].
After detection of PVT, an upper endoscopy should be performed to assess the presence and size of esophageal varices and/or gastric varices, and if required, primary or secondary prophylaxis of variceal bleeding should be initiated promptly prior to anticoagulant therapy [11].
Treatment
After confirming the diagnosis of nontumoral PVT and its extent and degree of luminal occlusion, it is important to stratify patients according to the possibility of undergoing liver transplantation, presence of concomitant PHT complications like variceal hemorrhage and refractory ascites, age, thrombocytopenia, and comorbidities including chronic kidney disease, in order to individualize clinical management decisions that primarily involve anticoagulant therapy [26]. In potential candidates to OLT, the extent and degree of luminal occlusion in PVT have implications on liver transplant surgery strategy as well as postoperative morbidity and early mortality. The outcome of grade 1 PVT patients (<50% luminal occlusion) does not differ from non-PVT patients, but grades 2-4havepooreroutcomes [23]. This highlights the importance of 50% luminal occlusion cutoff to determine which patients should be promptly treated. Fortunately, majority of patients with cirrhosis have partial thrombosis of the PVT often causing <50% luminal occlusion and were transient and completely disappeared spontaneously during the follow-up in two-third of the patients as was shown in the study by Nery et al. [7].
The therapeutic options include anticoagulation with conventional anticoagulants or direct oral anticoagulants in compensated (Child-Pugh A) cirrhosis and low molecular weight heparin (LMWH) in advanced cirrhosis (Child-Pugh B and C). In patients with contraindications, adverse events related to anticoagulation, progression of PVT on anticoagulation, or that have concomitant clinical indication for it, and interventional radiology techniques such as transjugular intrahepatic portosystemic shunt (TIPS) and mechanical thrombectomy are recommended [2]. A multidisciplinary team involving gastroenterologists, imaging specialists, interventional radiologists, and surgeons could be useful to provide the best treatment option for patients with cirrhosis and nontumoral PVT [27].
Anticoagulation
The aim of anticoagulant therapy is to recanalize the thrombosed veins and prevent further extension of the thrombus and complications such as intestinal ischemia due to extension of the PVT into the SMV [7]. An upper endoscopy for screening of esophageal varices and gastric varices is recommended in all patients candidates for anticoagulant therapy, and when indicated, primary or secondary prophylaxis of variceal bleeding should be initiated prior to starting anticoagulation [4]. However, this recommendation is not consensual and recent practice guidelines from the AASLD suggest that endoscopic band ligation is safe under therapeutic anticoagulation, and therefore, anticoagulant therapy, if indicated, should not be delayed until varices are eradicated [22].
In patients with cirrhosis and PVT, especially in liver transplant candidates, anticoagulation is indicated for those who are symptomatic, have luminal occlusion of the PV trunk over 50%, have extension to the SMV, and do not meet these criteria at the time of diagnosis but show progression of PVT during the follow-up [2]. Patients with chronic occlusion of PV or cavernous transformation with collaterals may not have a clear benefit of anticoagulation in terms of recanalization of occluded segment of PV [22]. In patients with thrombosis involving <50% of the lumen of PV trunk and/or branches, follow-up abdominal ultrasound with Doppler is recommended every 3 months to detect progression or resolution of the thrombus [1].
The strategy and clinical decisions guiding management of nontumoral PVT in patients with cirrhosis are shown in Figure 3. Nonetheless, it is important to make individualized decisions, taking into account, ongoing alcohol abuse, compliance to treatment of complications of cirrhosis, patient fragility, and comorbidities such as chronic renal failure and the risk of fall [2].
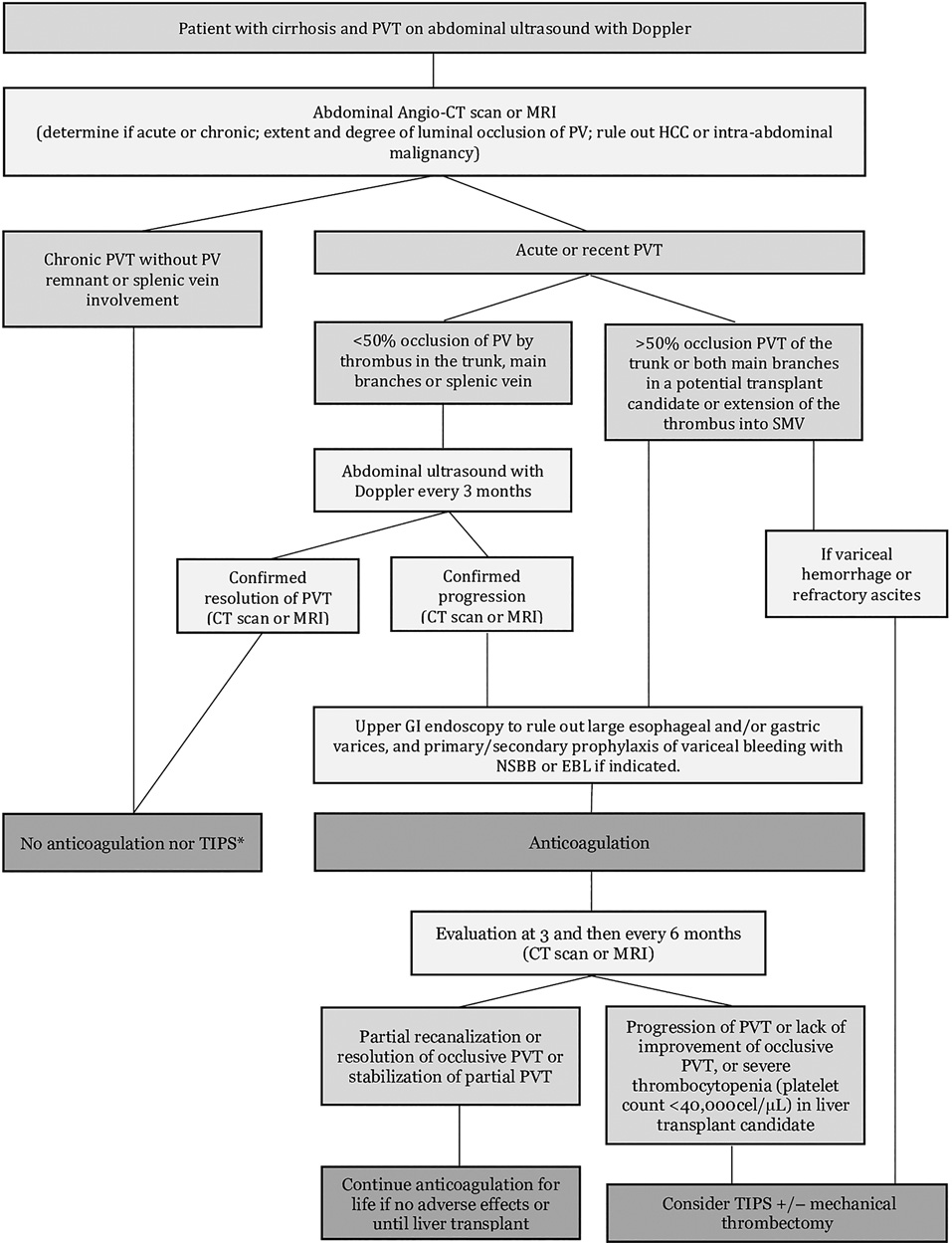
Fig. 3 Flowchart illustrating management of nontumoral PVT in cirrhosis. Adapted from Senzolo et al. [2].
The success of anticoagulation is correlated with early diagnosis of PVT and early initiation of anticoagulant therapy with patients that start anticoagulation within 6 months of detection of PVT being more likely to suc-cessfully recanalize PVT [28]. The pooled recanalization rates of PVT with anticoagulant therapy have been shown to be around 58% in a recent meta-analysis [29]. Factors like Child-Pugh class A, less extensive PVT, and absence of prior variceal bleeding appear to predict higher recanalization rates [30]. Anticoagulation significantly reduces all-cause mortality, compared to no treatment, and this benefitappears to be independent from PVT recanalization itself [29].
Anticoagulation appears to be safe in patients with cirrhosis when correctly selected, and a large systematic review showed that PVT patients treated with LMWH or vitamin K antagonists (VKA) achieved higher recanalization rates compared to placebo (72% vs. 40%), without significant differences in bleeding complications [31]. In the meta-analysis evaluating anticoagulant therapy in nontumoral PVT in cirrhosis by Guerrero A et al. [29], the bleeding rate was similar between the groups (anti-coagulation and no treatment), with no significant differences in PHT-related bleeding in the 2 groups of patients. The risk of bleeding appears to correlate with platelet counts at baseline <50,000 cells/µL, low serum albumin, and prior variceal bleeding [31].
The incidence of variceal bleeding in patients with cirrhosis and PVT was found to be significantly lower in those receiving anticoagulation compared to untreated ones (2% vs. 12%) [32], highlighting the benefit of anticoagulation in preventing future decompensations of cirrhosis. However, potential biases involving prophylaxis of variceal bleeding in patients receiving anticoagulation compared to untreated ones may have explained differences in variceal bleeding rates.
LMWH and VKA are considered as first choice in patients with cirrhosis and PVT, but the patient profile is extremely important [11]. LMWH is the initial treatment of choice at the standard dose of 1 mg/kg twice a day subcutaneously (with the necessary adjustments for kidney function). In patients with thrombocytopenia and renal failure, half the conventional dose of LMWH is often administered [4].
The main advantages of LMWH when compared to VKA are the fixed dose without the need for laboratory monitorization [33]. However, due to the need for parenteral administration and cost issues, oral options are normally preferred for long-term treatment [2]. VKA appear to be a safe option for Child-Pugh A and B (7 points). However, maintaining the international normalized ratio between 2 and 3 could be a challenge in these patients [2].
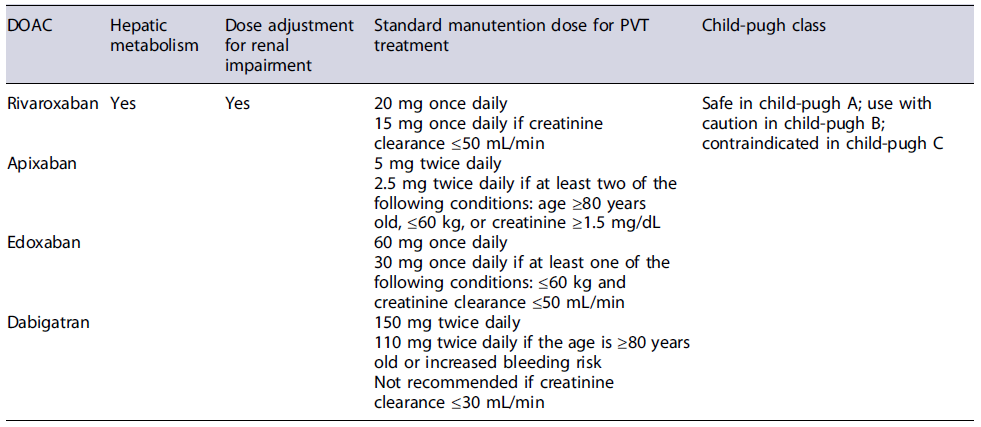
Direct oral anticoagulants (DOACs) are becoming an alternative to VKA [34]. However, despite several cohort studies confirming their efficacy and safety, there are still limited data from large studies regarding their safety in patients with cirrhosis as most of these patients were excluded from clinical trials [35]. In Child-Pugh class A patients, DOACs seem to be safe, but caution is needed in Child-Pugh class B patients and they are not indicated in Child Pugh class C [36]. Dose adjustment of DOACs is required in patients with renal impairment as shown in Table 1.
In patients with advanced cirrhosis (Child-Pugh B ≥8 points and Child-Pugh C), LMWH seems to be a safer option for long-term treatment [4]. During the course of treatment, an abdominal CT or MRI should be done after 3 months and then at 6 months to evaluate the efficacy of anticoagulation [1]. Anticoagulant treatment should be maintained for at least 6 months or indefinitely in patients on liver transplant waiting list and in those with SMV involvement [2]. The recurrence of thrombosis after stopping anticoagulant therapy in these patients is high with rates varying between 27% and 38%, and therefore, maintaining patients on anticoagulation should be considered especially in the absence of contraindications or adverse events [4, 29].
Thrombolysis
Thrombolysis, as used in other venous territories, has been reported to be effective in patients with cirrhosis who develop PVT, but significant procedure-related morbidity and mortality have been reported and there is no evidence that it is superior to anticoagulation alone in recent or acute PVT and therefore the most recent guidelines do not recommend this approach routinely [2]. In selected cases of recent PVT in whom intestinal ischemia persists despite anticoagulation, this approach could be an option [22].
Transjugular Intrahepatic Portosystemic Shunt
TIPS creates a low-pressure channel to bypass portal venous flow directly to the hepatic veins through a stent [11]. TIPS can be placed in a recanalized main PV or in a dominant collateral [37]. Often, the aim of TIPS is not to recanalize the thrombus, but to treat the PH-related complications due to it and guarantee the patency of portal tract [2].
TIPS may be useful in selected patients with PVT who have contraindications for anticoagulant therapy due to severe thrombocytopenia or in those who develop adverse events related to anticoagulant therapy, have progression of PVT on anticoagulation, and have PHT complications or no response after 6 months of anticoagulation [2, 38]. In some patients with cirrhosis, PVT is detected at the time of a clinical decompensation event such as acute variceal bleeding. The indication for TIPS placement in refractory
variceal bleeding or preemptive TIPS (Child-Pugh C <14 or Child-Pugh B >7 with active bleeding at index endoscopy or HVPG >20 mm Hg at the time of hemorrhage) is not affected in those patients who have concomitant PVT [39].
A recent meta-analysis demonstrated that TIPS for PVT recanalization was feasible, effective, and safe [38]. The technical success is around 95% [38], and failure to place TIPS was closely related to associated portal cavernoma and the degree of thrombosis within each venous segment of the portal venous system and extension into the SMV and splenic vein [37, 38]. In potential OLT candidates with chronic and complete PVT, PV recanalization and TIPS creation could also be considered to facilitate transplant eligibility [40]. Another key point in TIPS is the patency of the stent (around 89%), with a better performance with covered stents [38]. The use of a trans-splenic approach followed by TIPS creation in order to reestablish a patent main PV has been reported in a single-center case series [41].
There is controversy about post-TIPS anti-coagulation to prevent de novo PVT as well as to prevent thrombus extension and maintain the recanalization of PV achieved [42, 43]. In the meta-analysis by Guo et al. [44], post-TIPS anticoagulation was found to be associated with a higher risk of rebleeding. A recent randomized control trial suggested that TIPS placement alone can achieve high PVT recanalization rates possibly due to an increase in blood flow velocity in the splanchnic circulation [45]. However, the study results require validation and a risk-benefit evaluation should always be considered. We continue to favor anticoagulant therapy in extensive PVT especially when the SMV is involved.
The TIPS procedure is invasive, with risks that increase when this technique is combined with mechanical thrombectomy and direct catheter thrombolysis [38]. Also, post-TIPS cardiac dysfunction and encephalopathy are important safety concerns [46]. Post-TIPS encephalopathy has been reported to occur in 17-29% of patients [38].
The TIPS studies on PVT have some limitations. First of all, in most of the studies the indication for TIPS was PHT complications such as variceal hemorrhage and refractory ascites, and not the PVT itself, and most studies excluded chronic PVT, with an unknown impact on the technical success rates and adverse events [46].
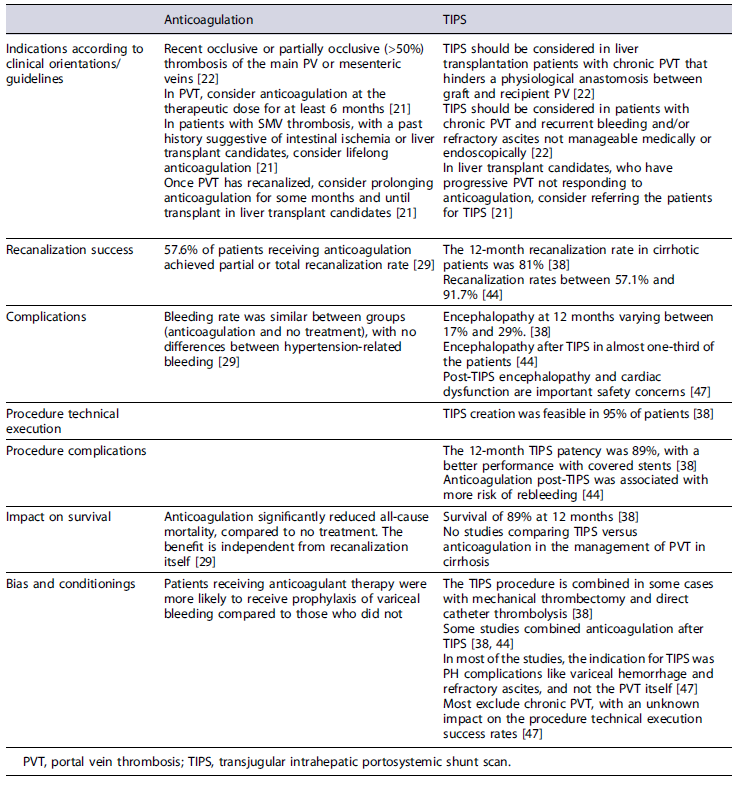
The differences in PVT recanalization rates and adverse events and limitations of anticoagulant therapy and TIPS placement for management of PVT are summarized in Table 2. After TIPS placement in patients with cirrhosis and PVT, a careful follow-up with scheduled TIPS venography in the first 2 months is crucial to access its patency and existence of residual PVT. Depending on the findings, interventions to perform additional PV recanalization or embolize spontaneous competing portosystemic shunts could be needed to maintain efficacy [40].
Table 2 Comparison of anticoagulant therapy versus TIPS in the management of nontumoral PVT in cirrhosis
Prognosis
The impact of PVT on hepatic decompensation and mortality in cirrhosis is still unclear. A large multicenter study showed that while the development of PVT correlates with the severity of liver disease at baseline, there was no association between PVT and progression of hepatic decompensation over time [7]. A small prospective randomized controlled trial comparing enoxaparin to placebo in the prevention of PVT in high-risk patients with cirrhosis without PVT at baseline showed not only efficacy in the prevention of development of PVT but also a higher survival and lower risk of decompensation in those who received enoxaparin during 1 year [48]. In this study, patients receiving anticoagulation were found to have lower rates of bacterial translocation and lower levels of inflammatory markers in the serum compared to those who did not receive anticoagulation [48].
There are no clear data about the impact of PVT on pretransplant mortality, with studies being contradictory [49, 50]. The same applies to posttransplant survival, with some studies showing no differences between patients with PVT and without and other studies showing higher morbidity and short-term mortality post liver transplant in those patients who had PVT at the time of liver transplantation [49, 51, 52]. In a recent study, comparing PVT recanalization or progression of the thrombus in cirrhosis, there were no differences observed between these patients in terms of 2-year survival, episodes of hepatic decompensation, hospital admissions, or need for liver transplantation [53].
In another study, in a subgroup analysis, any anticoagulant therapy in Child-Pugh B and C patients with PVT was associated with significant improvement in OLT-free survival [4]. In the same study, there was no overall difference in OLT-free survival in patients with any recanalization of PVT compared to no recanalization. These findings suggest that the potential benefit of anticoagulant therapy may go beyond macroscopic recanalization of PVT and may improve survival in patients with advanced cirrhosis [4]. Recently, in a meta-analysis by Guerrero et al.[29], anticoagulant therapy in nontumoral PVT in cirrhosis was found to significantly improve survival compared to no anticoagulation and this benefit was independent of the degree of PVT recanalization.
Conclusion
PVT is a common complication in patients with ad-vanced cirrhosis. Cross-sectional imaging with CT scan or MRI is essential to correctly determine the age of thrombus whether acute or chronic and the extent and degree of luminal occlusion in PVT and to rule out malignancy.
Anticoagulation is the cornerstone of PVT treatment especially in those patients who are candidates for liver transplantation, who are symptomatic, and in those with concomitant thrombosis of the SMV. Management decisions should be individualized after carefully evaluating potential risks and benefits of anticoagulant therapy. The risk of rethrombosis is not negligible, and the antico-agulant therapy should not be stopped, especially in liver transplant candidates. TIPS should be considered in patients who have contraindications to anticoagulation, have concomitant PH complications, develop adverse events, or have progression of thrombosis on anticoagulant therapy.