Introduction
Pediatric acute liver failure (PALF) is a rare and life-threatening multisystem and dynamic disorder that progresses to multiorgan failure within days or weeks in children with no pre-existing chronic liver disease [1]. Although the actual incidence of PALF is unknown, it is estimated to be the cause of liver transplantation (LT) in 10-15% of pediatric population undergoing LT [2, 3].
Etiology depends on age and epidemiology, and includes viral hepatitis, metabolic disorders, autoimmune hepatitis, ischemia, neoplastic disease, and toxins [4]. However, there are still many cases in which etiology remains indeterminate (40-50%) [1, 5]. Although its presence is not strictly necessary, unlike adults, most children develop hepatic encephalopathy with varying degrees of impairment [1], and its signs can be subtle.
Despite the advances in supportive measures and target treatment for specific etiologies in intensive care units [6], ALF carries a high mortality risk or requires LT in 70% of cases [2, 7]. Since that a critical factor in the prognosis of PALF is the early referral to a LT center [8], in January 2008, a multidisciplinary national meeting was held in Portugal on PALF, which involved the Societies of Gastroenterology, Hepatology and Nutrition and Pediatric Intensive Care of the Portuguese Society of Pediatrics, resulting in a consensus on action reinforcing the importance of its differentiated approach and early referral to the only one pediatric LT national center. Therefore, when patients have a PALF diagnosis in any hospital in our country, contact should be made with the LT center, in order to optimize its management and, eventually, provide an early hospital transfer [8].
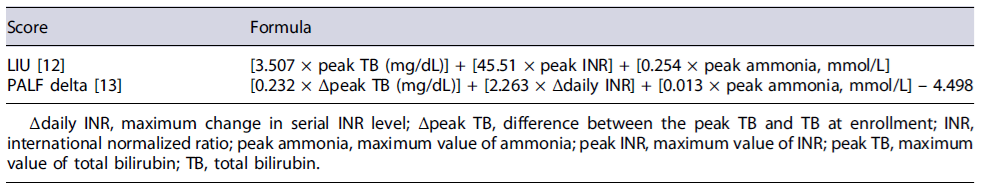
Several prognostic factors have been studied, including age, etiology, encephalopathy, serum bilirubin levels, coagulation factors (international normalized ratio [INR], factor V), ammonia, and serum lactate [9]. Scoring systems have also been developed to help predict the risk of death or the need for LT, trying to improve organ allocation decisions. The pediatric end-stage liver disease (PELD) for children aged less than 12 years has been used to predict mortality in children with a chronic liver disease listed for LT. However, its validity as a prognostic score in PALF is questionable, due to the limited use and non-consensual results [10]. Another one, the liver injury unit (LIU) scoring system, appears to predict the like-lihood of receiving a LT better than the risk of death [11, 12].
Most of these scoring models use static clinical param-eters assessed within the first hour or the first day, so they do not reliably predict mortality as the disease may rapidly deteriorate in hours or days [13, 14]. A new, non-yet validated prognostic score, the PALF delta score (PALF-ds), based on changes in serial laboratory values in the first week after admission, seems to have a higher predictive mortality accuracy, despite the complexity of its calculating formula [15].
The pediatric intensive care unit (PICU) scoring systems like pediatric risk of mortality (PRISM) and pedia-tric index of mortality (PIM) have been developed to assess the severity of illness and mortality risk, irrespective of the diagnosis but they have been scarcely studied in PALF [6, 16]. Currently, unlike adults, there are no validated PALF prognostic scores that clearly distinguish between patients who will recover spontaneously from those who will need LT to survive [3]. So, it is essential to establish more versatile, accurate, and straightforward prognostic tools. Our study aimed to investigate the accuracy of several biomarkers and scores as prognostic tools in PALF in a PICU of a national referral center for pediatric LT.
Materials and Methods
An observational study with retrospective data collection was performed.
Eligibility criteria were children and adolescents (aged between 0 and 17 years) admitted to a PICU of a national referral center for pediatric LT between January 1994 and January 2022 with PALF diagnosis from the PICU database. Patients with PALF due to secondary liver injury related to multipleorganfailurewereexcluded.
The sample was divided based on patient outcomes into two groups: spontaneous recovery (SR) which included patients benefiting from eventual etiologic treatments such as N-acetylcysteine therapy and non-SR (NSR - transplantation or death at PICU discharge). All patients benefitted from standard supportive care.
PALF was defined according to Pediatric Acute Liver Failure working group criteria as biochemical evidence of liver injury and coagulopathy not corrected by vitamin K in the presence of an INR greater than 1.5 in patients with hepatic encephalopathy, or an INR greater than 2, regardless of hepatic encephalopathy, in patients with no known evidence of chronic liver disease [4, 6]. To assess the presence and grade of hepatic encephalopathy, it used a scale adapted to pediatric age [17].
Data collection was obtained by consulting the computer records from the PICU and hospital general databases (B-ICU Care, SClinico) and, in older cases, from paper medical charts. The variables analyzed were age, gender, etiology, presence and grade of encephalopathy; biomarkers at admission, and peak values (alanine aminotransferase, aspartate aminotransferase, total bilirubin [TB], serum ammonia, serum lactate, INR, serum albumin, creatinine, sodium); and PICU support therapies (mechanical invasive ventilation, cardiovascular support, and continuous renal replacement therapy). The following prognostic scores were calculated (Table 1):
PELD/MELD scores. The PELD score was applied in patients aged less than 12 years, and the MELD score was applied in patients aged 12 years or older [10, 18] at the time of PICU admission using available internet calculator (peld-score-pediatric-end-stage-liver-disease-younger-12 and meld-score-model-end-stage-liver-disease-12-older).
LIU score at PICU admission according to the formula described in Table 1 [12].
PALF-ds was obtained from the laboratory values at PICU admission and its evolution over the following 7 days [14]. The PICU mortality prediction model, PRISM and PIM, scores were also considered. The PRISM score uses fourteen physiologic variables (blood pressure, heart rate, respiratory rate, PaO2/FiO2, PaCO2, Glasgow coma score, pupillary reactions, coagulation study, TB, potassium, calcium, glucose, and bicarbonate) and is automatically calculated by completing a patient’s clinical situation form in the first 24 h of PICU admission. Similarly, PIM score uses ten variables (systolic blood pressure, pupillary reaction, PaO2,FiO2, mechanical ventilation, base excess, elective/urgent ICU admission, reason for ICU admission, and risk diagnosis) that were obtained by filling the clinical form with clinical admission data.
Statistical analysis was performed with the Statistical Package for the Social Science, version 27. Nominal variables were expressed as numbers and percentages. Numeric variables were reported with mean and standard deviation or median and interquartile range (P25 to P75), depending on its distribution.
The χ2 test or Fisher’s exact test, according to Cochran rules, was used to compare nominal variables. Regarding quantitative variables, comparisons between groups were made, using the parametric test, independent sample T test and the nonparametric Mann-Whitney U test, as appropriate. The threshold for significance was defined as p < 0.05.
The receiver operating characteristic curve of all the prognostic scores and biomarkers considered was analyzed. We used the area under the receiver operating characteristic curves (AUC) as an effective way to evaluate the overall diagnostic accuracy of the score/biomarker [19]. So, an AUC of 0.5 suggests no discrimination capacity of the model; 0.7 to 0.8 is considered acceptable; 0.8-0.9 is considered excellent, and more than 0.9 is considered outstanding [19].
We performed a multivariate analysis through a logistic regression model to evaluate the influence of some variables on lactate capacity to predict NSR. Omnibus Test of Model Coefficients and Nagel R Square statistic was applied to assess good fit. The good fitqualityof the model was classified according to Nagelkerke R2:poorquality <10%, moderate quality 10-50%, good to very good quality >50%. Results of the logistic regression analysis were reported as adjusted odds ratios with 95% confidence intervals (CIs).
Results
The study included 59 patients with a median age of 24 months (interquartile range 4-68), 54% of the female gender. According to admission year, 35 patients were admitted between 1994 and 2007 and 24 between 2008 and 2022.
There were 53 children transferred from other hospitals. The median time between the onset of symptoms and PICU admission was 5 days (2-14).
The most frequent etiologies were metabolic (N = 15; 25.4%) and infectious (N = 11; 18.6%); in 32.2% (N = 19) of the patients, it was not possible to determine an ethology. SR occurred in 21 (35.6%), and NSR occurred in 38 patients (64.4%).
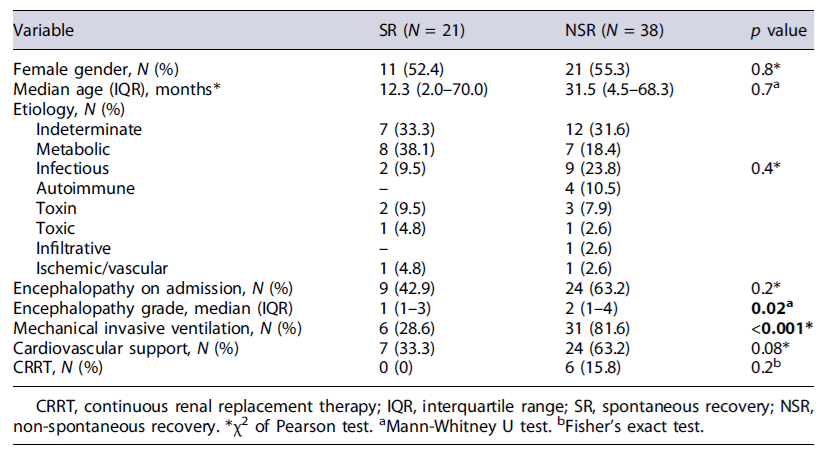
In the group of NSR, 25 children underwent LT (42.4%) and 19 died, of whom six after LT. From the remaining 13 that died, two were considered for LT. Table 2 shows the demographic and clinical characteristics of the two groups.
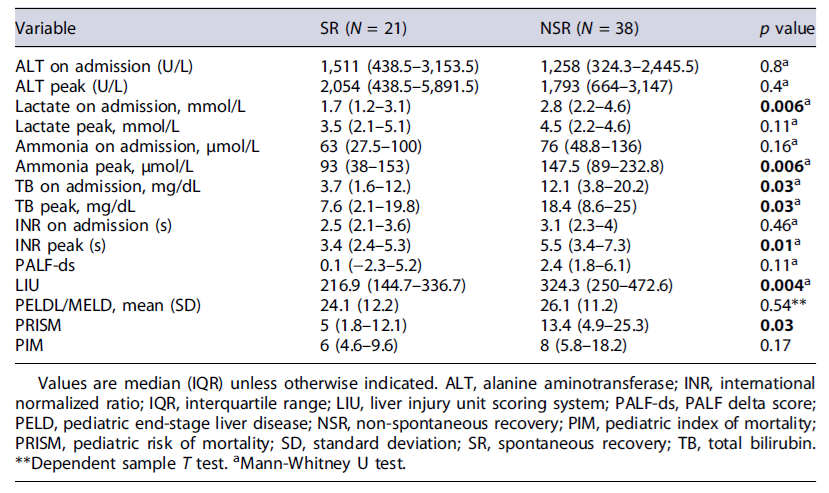
More than half (N = 33; 55.9%) had encephalopathy on admission, with the highest grades in the group of NSR (p = 0.02). Patients in the NSR group often required mechanical invasive ventilation (p < 0.001), cardiovascular support, and continuous renal replacement therapy. Biomarkers and scores were compared between the two groups (Table 3). Lactate and TB on admission, ammonia peak, TB peak, and INR peak were significantly higher in the NSR group than in the SR group.
The NSR group had higher values in all prognostic scores analyzed. However, these differences were only statistically significant in LIU and PRISM scores.
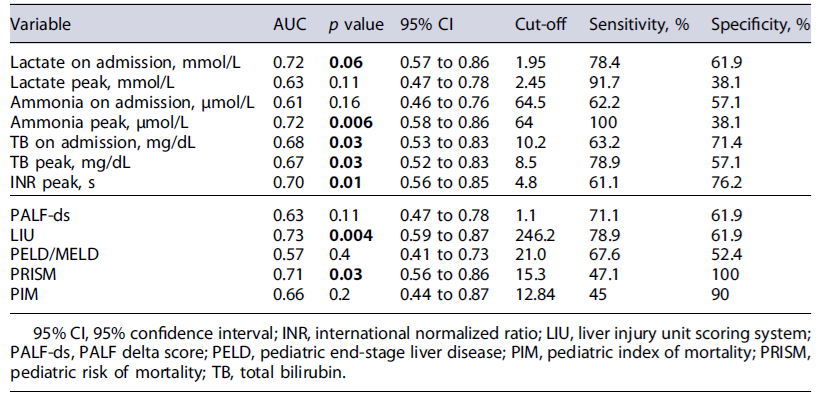
The accuracy of the biomarkers and scores to predict NSR can be observed in Table 4. NSR was acceptable for lactate at admission (AUC 0.72; 95% CI: 0.57-0.86; p = 0.006), ammonia peak (AUC 0.72; 95% CI: 0.58-0.86; p = 0.006), and INR peak (AUC 0.70; 95% CI: 0.56-0.85; p = 0.01).
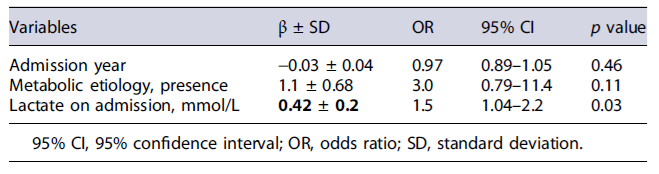
Additionally, two prognostic scores had acceptable discrimination performance for NSR with AUC 0.73 for LIU and 0.71 for PRISM, both statistically significant. The multivariate analysis with a logistic regression model was statistically significant (X2 [3] = 11.8, p < 0.001). The model correctly classifies 88.7% of the cases and has a moderate quality of good fit (Nagelkerke R2 24.1%)(Table 5). The lactate on admission was an independent factor for NSR.
Discussion
PALF treatment involves the challenging decision whether to maintain only supportive medical therapy or to advance to an urgent LT. LT is the only proven treatment for PALF and has allowed, in recent decades, a significant improvement in its prognosis, increasing survival to about 60-85% [8].
Despite these encouraging results, optimal prognostic criteria are lacking. An effective prognostic model for PALF is essential for clinical practice to accurately differentiate between patients who may recover spontaneously from those who probably will die without LT [3]. This process would help with appropriate organ allocation, avoiding unnecessary LT in patients with a good chance of SR [3].
Another critical factor in the prognosis of PALF is the early referral to a LT center [8]. The multidisciplinary and differentiated approach allows optimization of support measures, undergoing LT in better conditions, identifying situations in which LT is contraindicated and eventually enhancing the probability of SR. An early referral to the LT center is associated with higher survival than a late referral [8].
Developing prognostic models or scores for PALF encompasses numerous challenges. In particular, the heterogeneity of age and etiology in PALF, and a lack of universal understanding of the natural history of the disease, poses additional difficulties, not permitting the application of adult criteria.
Several laboratory variables have been identified, and their incorporation into scoring systems has been attempted, but no optimal validated model for PALF has yet been established. A suitable model should reflect the dynamic nature of PALF and simultaneously include easily accessible variables, making it easy to apply in clinical practice.
In the present study, the authors evaluated the prognostic accuracy of several biomarkers and scores at admission and during the first week in children with PALF admitted in a PICU to predict the outcome, namely, death or need for LT (NSR). Regarding the etiology of PALF, metabolic disorders were the most frequent (25.4%), possibly because our hospital is a reference for this type of disease. Metabolic conditions account for approximately 10% of all cases of PALF and 18% of PALF cases among children younger than 3 years [8].
The proportion of patients with indeterminate etiology (32.2%) is lower than described in most studies [1, 2, 7]. These numbers may be related to a large diagnosis effort based on an exhaustive investigation, even after patients’ death.
This center is the Portuguese reference in pediatric LT and PALF since 1994 and 2008, respectively, with a reduction in referral time and severity of cases upon admission since 2008 and a trend to mortality decline [20]. Thus, our results are in line with the noteworthy mortality associated with PALF (32.2%) and LT survival rate (76%), as described in the literature [1, 2, 4].
The majority of our patients had encephalopathy, which was more severe at admission in the NSR group. The presence of encephalopathy is widely associated with poor outcome [1, 3, 20] in particular in adult ALF. However, encephalopathy is challenging to incorporate into a prognostic model as it can be subtle and difficult to evaluate in younger children [17].
Several prognostic and scoring systems for adult ALF have been suggested, but there is a limitation to the applicability of these prognostic scores to PALF, as pointed above [3, 14]. Given the severity and dynamic of this condition, robust prognostic tools with reasonable accuracy to early identify patients with a poor prognosis to include them in the urgent transplant list are needed [1]. As most of the decisions to LT listing occur within the first few days of admission, the evolution of biomarkers, highlighting the peak values, in the first week of hospitalization was considered.
These data indicate that lactate and TB on admission, peak values of ammonia, TB, and INR were significantly associated with NSR. All these values presented an ac-ceptable predictive accuracy with AUC ranging from 0.67 to 0.72, emphasizing the good discrimination power of lactate on admission and peak ammonia (both had an AUC value of 0.72).
Our study is one of the few studies that analyzed the accuracy of serum lactate as an isolated biomarker related to the evolution of PALF. The lactate value on admission had higher predictive power than the described scores, particularly the PALF-ds, characterized by high complexity in its calculation. Serum lactate as a prognostic tool has been studied in various situations of critically ill pediatric patients, such as septic shock[21]and has been included in PICU mortality scores [22, 23]. In some studies, a high blood lactate level at admission has been considered independently associated with and predictive of in-hospital mortality in the general population of critically ill children [22-24]. These results described in the literature support the relation between lactate and mortality that can be eventually applied in PALF. Therefore, the cut-off value for lactate of 1.95 mmol/L (with a sensitivity of 78.4% and specificity of 61.9%) seems relevant and helps identifying the patients that will not recover spontaneously. Additionally, lactate on admission was independently correlated with NSR when included in a logistic regression model with possible confounding factors, such as metabolic etiology.
Moreover, these results show that blood ammonia levels, especially peak values, could predict outcomes in PALF. Several studies found a significant difference on admission and peak biomarker values with definitely higher values in the group with organ loss, particularly for ammonia [1, 3, 15]. Liu, et al. observed in their cohort of 81 children with PALF that high ammonia levels were significantly associated with death or need for LT [11, 12]. Furthermore, compared with adult ALF, ammonia seems to be a far better prognostic parameter for children than for adults [1, 3, 15].
From the prognostic scores, LIU was the one that best predicted the evolution of both the need for LT and mortality in our study. This score is a tool that includes TB, INR, and ammonia peak values, significantly associated with death/LT in previous studies [11, 12]. Nevertheless, the predictive capacity (AUC 0.73) observed by us was lower than that found by other authors, which may be explained by the small sample size performed at a single institution.
The promising new dynamic, PALF-ds score based on changes in serial laboratory data using the variations of TB/INR to predict NSR had AUC of 0.63 (sensitivity of 71.1% and specificity of 61.9%), lower than some laboratory isolated variables. Moreover, it had lower predictive performance than described by its authors (AUC 0.918; sensitivity 81%, specificity 91% to predict death) [14]. This discrepancy may be explained by the lack of external validation of the score, which was only applied to two populations of South Korea different from ours (lower proportion of metabolic etiology and a higher percentage of undetermined etiology). Furthermore, the authors considered different outcomes compared with our study, analyzing three groups: LT, death, and SR. Another contributing factor may be the influence of the score’scomplexity, including several evolutive values, which increase the probability of missing data on a retrospective study.
The PELD/MELD score had poor prognostic accuracy in PALF outcome, according to what is described in the literature. King’s College Hospital Criteria (KCH criteria) and Clichy-Villejuif Criteria, two adult most recognized prognostic models, do not adequately discriminate the patients who would die without LT, have low sensitivity, and do not reliably predict death in PALF [2, 25]. However, it is well established that a significant increase in INR/decrease in factor V level such as higher degrees of encephalopathy are associated with worse prognosis in PALF.
This study also has an innovative aspect regarding commonly used pediatric intensive care scores applied to the PALF setting. In this work, we observed that PRISM, a score based on age-related physiological parameters collected during the first 24 h after admission, had a good diagnostic accuracy with AUC 0.71 (p = 0.03). As mentioned in some studies, the PIM score had a lower predictive power in PALF [22, 23, 26].
The limitations of our study include its retrospective nature, the small sample size, and the specific population analysis, which differs from the other studies. The ex-tended study period increased the probability of bias and missing data since we could not account for undocumented data in the paper support since the beginning of LT in Portugal. Moreover, a referral bias may exist as our institution is a LT and PALF referral center.
In conclusion, PALF is a rare, highly heterogeneous, and progressive disease. To date, no optimal PALF prognostic model exists. Prognostic variables in PALF are determinants for emergency LT allocation, so they must be dynamic but at the same time easy to measure and unbiased to interpretation [25]. Although more research is needed, especially through the construction and analysis of multicenter international databases, our study highlights that the blood lactate level upon admission and peak ammonia is robust, simple, direct, and accurate predictors of poor outcomes in PALF. Furthermore, these results lead to the possibility of considering the inclusion of lactate in the prognostic scores, particularly in LIU score.