Introduction
Liver cirrhosis is a consequence of chronic liver inflammation followed by the development of diffuse fibrosis and the growth of regenerative nodules [1]. It is one of the most common diseases worldwide and the 11th leading cause of death, ranking as the 3rd cause of death in patients aged 45-64 years [1, 2].
The main causes of liver disease are alcoholic liver disease, metabolic dysfunction-associated steatotic liver disease (MASLD) and viral hepatitis (hepatitis C [HCV] and hepatitis B). However, due to effective treatment of HCV and widespread vaccination strategies for hepatitis B, MASLD is becoming the primary cause of liver disease and is soon expected to be the leading cause of cirrhosis, hospitalization and liver transplant [3-5].
Cirrhosis is clinically classified into two prognostic stages: compensated cirrhosis (or compensated advanced chronic liver disease), when there are no episodes of decompensation, with a median survival of more than 12 years; and decompensated cirrhosis, based on the presence of complications related to portal hypertension (PH), with a contrasting median survival of 2 years [3, 6, 7]. Portal pressure results from two independent factors, as stated by Ohm’s law:
In the early stages, the primary factor contributing to an increase in portal pressure is resistance caused by growing fibrosis in the liver. In more advanced phases, augmented flow due to splanchnic vasodilation, angiogenesis, and the development of portosystemic collaterals become the main contributors [8].
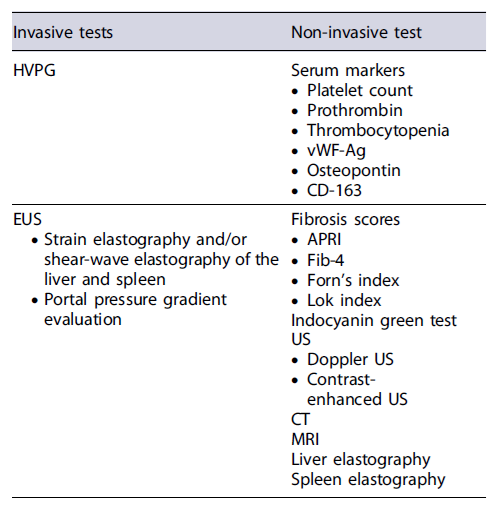
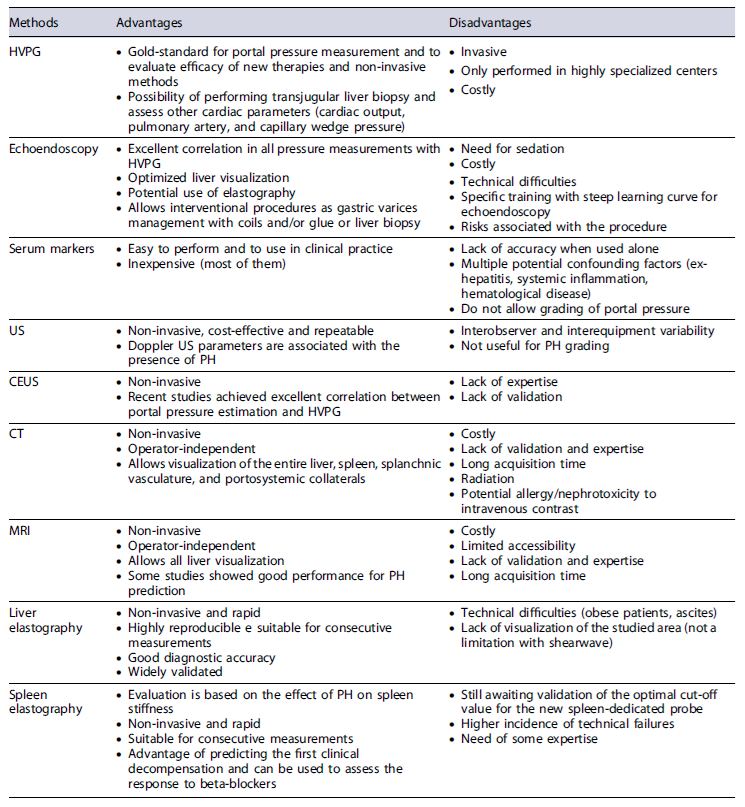
PH is defined as an increase in the pressure between the portal vein and the hepatic veins (systemic venous pressure) above the normal level (1-5 mm Hg) [9]. Clinically significant portal hypertension (CSPH) is defined by a hepatic venous pressure gradient (HVPG) ≥10 mm Hg and is independently associated with the development of severe complications, including ascites, bleeding from gastroesophageal varices, hepatic encephalopathy, hepato-renal syndrome, and spontaneous bacterial peritonitis [3, 6, 10]. CSPH is present in all patients with decompensated cirrhosis and is associated with a 1-year mortality rate of 20% (vs. 5.4% in patients with compensated cirrhosis) [3, 6, 10]. Therefore, recent Baveno VII guidelines emphasize the importance of an early diagnosis of CSPH; the presence of CSPH will lead to the early initiation of carvedilol as it can significantly impact the prognosis, reducing the rate of cirrhosis decompensation [11, 12]. The aim of this review was to evaluate the non-invasive and invasive techniques for assessing the presence of PH (Tables 1, 2, 3).
Non-Invasive Assessment of PH
Serum Markers
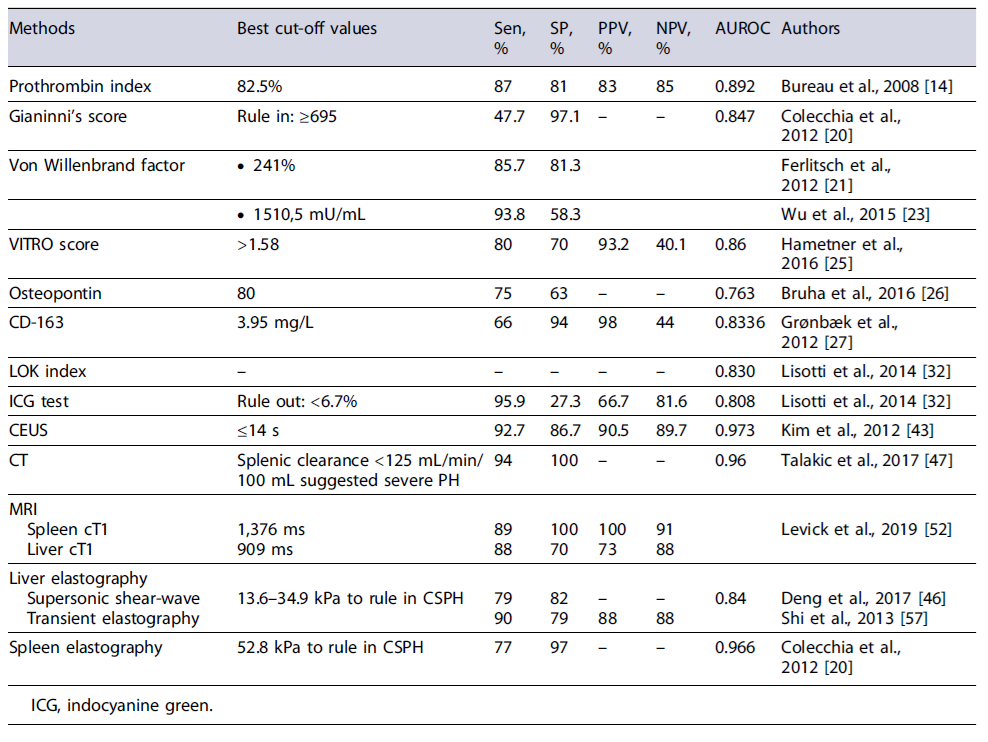
Serum markers have long been tested for the detection of CSPH but have only shown moderate results. Platelet count and prothrombin levels are the most commonly studied biochemical parameters that are not sufficiently accurate to be used in clinical practice. The prothrombin index showed a good performance (AUROC of 0.89) for the diagnosis of CSPH, but it may be strongly affected by other external factors such as hematological diseases or coagulopathy [13, 14]. Thrombocytopenia is a common complication in cirrhotic patients but is a complex and multifactorial phenomenon, being not only associated with spleno-megaly but also with decreased hepatic production of thrombopoietin and increased platelet destruction [15]. No specific platelet value has been found to correlate with the presence of esophageal varices or changes in portal pressure. However, in combination with spleen diameter (measured by ultrasound [US]) - the Giannini’sscore- a good correlation with HVPG was found, though lower than with liver elastography (AUROC 0.847 vs. AUROC 0.899) [3, 13, 16-20].
Von Willebrand factor antigen (vWF-Ag) is an indicator of endothelial activation and is frequently elevated in cirrhosis [21]. Ferlitsch et al. [21] first proposed vWF-Ag as a serum marker predictive of CSPH; using a cut-off of ≥241%, it showed an AUROC of 0.85 for predicting CSPH in compensated patients [21-23]. A systematic review and meta-analysis revealed that it was moderately correlated with HVPG measurement [24]. A more recent study, the VITRO score (vWF-Ag/thrombocyte ratio), found an AUROC of 0.86, higher than vWF-Ag alone [25]. Osteopontin is an extracellular matrix protein associated with inflammation and fibrosis and was shown to be related to the degree of fibrosis in liver disease. However, a study including 157 cirrhotic patients only showed a fair predictive capacity of osteopontin at a cut-off of 80 ng/mL for prediction of CSPH (AUROC of 0.763) [22, 26].
CD-163 level was also shown to be predictive of the degree of HVPG. A study by Grønbæk et al. [27] found a good capacity for predicting the presence of PH (AUROC 0.8336), and, in association with enhanced liver fibrosis score (which includes hyaluronic acid, tissue inhibitor of metalloproteinase-1, and pro-collagen III), was able to identify PH with an AUROC of 0.91 [27-29].
Several fibrosis scores were also evaluated for the diagnosis of PH. The AST/platelet ratio index (APRI), fibrosis-4 (Fib-4), and Forn’s index are used to grade fibrosis but showed limited accuracy for the diagnosis of CSPH [30]. Procopet et al. [31] and Lissoti et al. [32] found that the Lok index (derived from AST/ALT ratio, prothrombin index and INR, and platelet count) was predictive of CSPH and esophageal varices [3, 31, 32]. The indocyanine green retention test is used to assess liver function in patients proposed for liver surgery. A study showed that indocyanine green 15-min retention test could rule out CSPH (AUROC = 0.808) [32].
However, none of these tests, when used alone, have enough accuracy to be used in clinical practice. Therefore, the definition of algorithms and combination of tests are strongly advised [13].
Ultrasound
US is the primary non-invasive method used for diagnosing cirrhotic patients and screening for hepato-cellular carcinoma (HCC). It is a cost-effective, non-invasive, and repeatable procedure. The most common US findings suggestive of liver cirrhosis are nodular surface and splenomegaly [13].
US can also be utilized for predicting the presence of CSPH. Berzigotti et al. [33] conducted a study and found that spleen diameter was the only US finding associated with CSPH (sensitivity 93% and specificity 36%), albeit only in the univariate analysis [13, 33]. However, it is not a specific indicator, as an enlarged spleen can also be observed in certain hematologic and infectious diseases.
On the other hand, the presence of portosystemic abdominal collaterals is a specific sign of PH and has been linked to a higher risk of first decompensation [34, 35].
Dilatation of the portal vein >13 mm (sensitivity <50%and specificity 90-100%), splenic vein and superior mesenteric vein (sensitivity 72% and specificity 100%), as well as reduced respiratory variation (sensitivity 79.7%and specificity 100%), are highly specific indicators but lack sensitivity for detecting PH.
Several doppler US parameters are associated with the presence of PH, including reduced portal vein velocity, reversal of portal blood flow, and increased intraparenchymal hepatic and splenic artery resistance and pulsatility index [36-38]. One study found that splenic arterial resistance index outperformed liver stiffness measurement (LSM) by shear-wave elastography in identifying PH [39]. However, doppler US has not been proven effective for the grading of PH, and variability between different diagnostic centers, as well as interobserver and interequipment variability, can limit its comparability [40-42].
Contrast-enhanced US, initially developed for evaluating the vasculature of focal lesions, has shown promising results in assessing PH. Kim et al. [43] used CEUS for measurement of hepatic vein arrival time and showed a very good correlation with the presence of CSPH. Eisenbrey et al. [44] also demonstrated a good correlation between HVPG and the use of subharmonic aided pressure estimation using perflubutane micro-bubbles. More recently, Berzigotti et al. [45] conducted the CLEVER study, using dynamic contrast-enhanced US with continuous infusion of SonoVue, and achieved an excellent correlation between portal pressure estimation and HVPG. Therefore, in the near future, contrast-enhanced US could potentially serve as a new tool for evaluating HVPG.
Computed Tomography and Magnetic Resonance Imaging
Cross-sectional imaging methods are commonly used in patients with cirrhosis, primarily for the evaluation of suspected HCC lesions detected in US. A meta-analysis reported a sensitivity of 87% and specificity of 88% for computed tomography (CT) scan to predict the presence of high-risk varices. Additionally, a small study involving 21 cirrhotic patients evaluated by CT perfusion imaging demonstrated excellent performance in detecting HVPG ≥12 mm Hg, with a sensitivity of 94% and specificity of 100% [46, 47].
Magnetic resonance imaging (MRI), due to its enhanced visualization capabilities and direct assessment of hemodynamic collateral circulation, has also been utilized to predict the presence of PH in cirrhotic patients [48]. In a retrospective study involving patients with chronic liver disease, relative liver enhancement and portal vein hyperintensity on a 20-min delayed T1-weighted gadoxetic acid-enhanced MRI were found to be predictive of HVPG ≥12 mm Hg [49]. Another study revealed a correlation between spleen volume, spleen stiffness, and liver stiffness measured by MR elastography, as well as the presence of varices [50]. Additionally, a noteworthy study demonstrated the ability to differentiate healthy individuals from patients with PH by combining hepatic architecture and splenic artery velocity obtained by MRI [51]. Levick et al. [52] also showed that cT1, a novel MRI-based quantitative metric for assessing a composite of liver inflammation and fibrosis, mainly spleen cT1, correlated well with the presence of ph.
However, high costs and limited accessibility (mainly MRI), lack of validation and expertise for both techniques, long acquisition time, and radiation exposure (in case of the CT scans) are major drawbacks for their routine use in clinical practice.
Liver Elastography
Transient elastography is an US-based technique used to assess liver fibrosis. It measures the speed of a wave propagated by a probe, which is directly proportional to liver stiffness. This technique has been a major break-through in the field of hepatology in recent years and has become a routine part of hepatologists’ daily practice for evaluating fibrosis and diagnosing patients with liver cirrhosis [13].
The first study to evaluate the relationship between LSM and HVPG was published in 2006. It demonstrated a direct association between LSM (a cut-off value of 8.7kPa) and the diagnosis of PH (defined as HVPG ≥6) in patients with HCV cirrhosis after liver transplantation, with an AUROC of 0.93 [53]. Since then, several studies have been published, demonstrating the high sensitivity of transient elastography. Patients with LSM values up to 13.3 kPa have a very low risk of CSPH, while those with LSM values of at least 21.1 kPa have a very high risk of CSPH [13, 54-57]. However, LSM values in the intermediate range, referred to as the “gray zone,” do not allow for an accurate diagnosis [13]. LS measurement has also been used to predict the presence of esophageal varices, which indirectly evaluate PH. A meta-analysis showed a sensitivity of 0.87 and specificity of only 0.59 for the detection of large varices when using LSM as the sole method. Moreover, when LS measurement is used alone, 30% of cases fall into the gray zone, resulting in an inaccurate diagnosis [13]. To address this limitation, several authors have suggested combining two non-invasive methods. The varices risk score, which incorporates LSM, platelet count, and spleen diameter, has shown an AUROC of 0.909, providing improved accuracy [16].
More recently, Baveno VII guidelines have placed emphasis on the use of non-invasive tests for the diagnosis of CSPH in clinical practice [11]. According to these guidelines, for patients with LSM ≤15 kPa and platelets ≥150 × 109/L, CSPH can be ruled out with high sensitivity and a negative predictive value (>90%) [11].
On the other hand, in patients with viral cirrhosis, alcoholic cirrhosis, or non-obese MASH cirrhosis, a LSM ≥25 kPa indicates the presence of CSPH with high specificity and a positive predictive value (>90%) [11].
For patients with MASLD cirrhosis, further validation is required to determine the appropriate cut-offs, as a high body mass index (especially if ≥ 30 km/m2) is associated with higher LSM, regardless of the risk of CSPH [11, 58].
An interesting finding is that LSM is effective in predicting HVPG values up to 10-12 mm Hg, but its predictive accuracy decreases for higher values above. This observation can be explained by the fact that in the early phases of PH development, the main contributing factor is the accumulation of fibrillar extra-cellular matrix and fibrosis, which can be detected by LSM. However, in more advanced stages, the primary factors are hyperdynamic circulation and splanchnic vasodilatation, which are not effectively evaluated by LSM [13].
One of the major drawbacks of LSM using transient elastography is its limited control area of interest, as it provides a monodimensional view [59]. Shearwave elastography (two-dimensional or point shearwave), on the other hand, is a method incorporated into more advanced US devices that enables direct visualization in the region of interest and provides a quantitative measurement of liver stiffness in kPa. A meta-analysis of four studies demonstrated promising performance of shearwave elastography for diagnosing CSPH with an AUROC of 0.84, sensitivity of 79%, and specificity of 82% [60]. At the cut-off of 24.5 kPa, shearwave liver elastography showed an AUC of 0.87 for the diagnosis of CSPH [61]. Shearwave elastography has improved the accuracy of elastography in challenging patient populations (such as those with ascites or obesity), but it also faces some challenges that hinder its routine implementation. These challenges include the need for experience performing US examinations, variability in values obtained from different US devices, and variations in elastography techniques, all of which affect the definition of cut-off values and the accurate interpre-tation of results [8, 62, 63].
Spleen Elastography
Spleen stiffness measurement (SSM) was first described by Stefanescu et al. [64] in 2011 as a method for evaluating PH. Initially, the SSM value of 56 kPa was associated with CSPH. However, subsequent studies have shown that lower values (≤41-46 kPa) can effectively rule out the presence of CSPH and high-risk varices [20, 65, 66]. A meta-analysis of nine studies demonstrated good accuracy of SSM in detecting PH (SROC of 0.92, sensitivity of 88%, and specificity of 84%). When compared to HVPG, SSM showed a strong correlation with values >5 mm Hg and with the progression of PH from earlier to late phases [20, 66].
Theoretically, SSM is considered superior to LSM for evaluating PH because it not only reflects the increased hepatic resistance due to liver fibrosis (which LSM also assesses) but also captures the heightened hyperdynamic circulation and splanchnic vasodilatation that occur in more advanced phases of PH [67]. Recent comparative data supports the notion that SSM may be a superior marker of PH compared to LSM, not only for viral liver disease but also for other etiologies. In a meta-analysis that included patients with chronic liver disease, SSM demonstrated superior sensitivity and specificity compared to LSM (0.88 vs. 0.83 and 0.78 vs. 0.66, respectively) for the diagnosis of esophageal varices, an indirect marker of PH [68]. SSM also offers the advantage of predicting the first clinical decompensation and can be used to assess the response to β-blockers [69-71]. However, SSM has certain limitations, including a higher incidence of technical failures compared to LSM, particularly in cases involving obesity, ascites, or invalid SSM. Additionally, previous studies utilized probes with an upper limit cut-off value of 75 kPa, which is now considered for spleen stiffness, as spleen stiffness typically exhibits higher values. The development of a novel spleen-dedicated stiffness measurement probe, capable of reaching 100 kPa, has improved the accuracy of SSM and addressed this limitation [3, 72].
SSM has gained significant importance in recent years and is now recommended in the Baveno VII guidelines. According to these guidelines, an SSM value of <21 kPa can effectively rule out CSPH, while an SSM
value >50 kPa can indicate the presence of CSPH in cases of viral hepatitis [11]. However, it is worth noting that the validation of the optimal cut-off value for the new spleen-dedicated probe is still pending. Further studies and validation are needed to determine the most appropriate cut-off value for SSM using the new probe [11].
Invasive Assessment of PH
Hepatic Venous Pressure Gradient
Hepatic vein catheterization is the gold standard for measuring HVPG and determining the portal pressure. HVPG is calculated as the difference between wedge hepatic venous pressure (WHVP) and free hepatic venous pressure [6, 9].
Procedure
The procedure is performed under sedation and local anesthesia. The right jugular vein (or the femoral vein) is catheterized, usually with US guidance, and a balloon-tipped catheter is passed into the hepatic vein under fluoroscopic guidance [9, 22].
The free hepatic venous pressure is measured in the hepatic vein, 2-4 cm from its opening into the inferior vena cava, and should be similar to the value obtained in the inferior vena cava; a difference of >2 mm Hg can occur due to inadequate placement of the catheter or, less commonly, due to a hepatic vein obstruction [9, 22]. The WHVP is measured by inflating the balloon in the hepatic vein until total occlusion; adequate occlusion could be confirmed by injection of contrast dye without observing reflux.
Occluding the hepatic vein stops the blood flow, equalizing the pressure in the vascular territory, specifically in the hepatic sinusoids. Therefore, the WHVP reflects the pressure in the hepatic sinusoids, which is slightly lower than the portal pressure (~1 mm Hg) in a normal liver. Contrarily, in cirrhosis, the blood flow cannot be decompressed at the sinusoid level due to fibrosis and nodule formation, making the WHVP an accurate estimation of portal pressure [9, 73-75].
Complications
Complications during HVPG measurement are un-common. Local injury at the puncture site, such as leakage, hematoma, or arterial-venous fistula, is one of the most frequent complications, but the risk can be reduced with ultrasonographic guidance. Self-limited arrhythmias can occur during the passage of the catheter through the right atrium; carbon dioxide can be used in patients allergic to contrast. The risk of bleeding is very low, and platelet or fresh frozen plasma transfusion before the procedure should only be considered in cases of severe thrombocytopenia (platelet level <20 × 109/l) or low prothrombin ratio (<30%) [9, 22]. Although safe, HVPG measurement is an invasive procedure, costly, only performed in highly specialized centers, and unsuitable for consecutive measurements during the course of the disease [3].
Endoscopic Ultrasound
Endoscopic ultrasound (EUS) is an established diag-nostic and interventional tool for biliary diseases. In recent years, EUS has gained importance in patients with liver disease, from assessing small liver lesions to evaluating and treating esophageal varices (EV) [76]. The concept of endohepatology has emerged recently as a field where EUS plays a key role in the diagnosis and management of liver diseases. EUS has shown promising results in the management of patients with PH as it is associated with optimized liver visualization, the potential use of elastography, and allows interventional procedures such as gastric variceal treatment (coils and/or glue), liver biopsy, and portal pressure measurement with very good results [77].
Through the esophageal, gastric, or duodenum window, EUS has a wide visualization of the entire liver, biliary tree, and pancreas, as well as direct sonographic visualization, which increases the diagnostic success (bigger liver specimens when compared to percutaneous liver biopsies) and reduces the adverse event rates of liver biopsy (approximately 2.5%) [78, 79].
EUS can also have a predictive role in recurrent EV bleeding. A study involving 206 cirrhotic patients with previous variceal bleeding found that the presence of large peri-esophageal collateral veins and perforating veins were significant factors for recurrent EV [80, 81]. Another study also demonstrated that EUS can predict the risk of annual bleeding based on variceal cross-sectional surface area [82].
EUS-guided portal vein catheterization with portal pressure gradient measurement has also been proposed. One of the first studies was performed in three swine models, showing excellent correlation in all pressure measurements when compared with EUS measurements with HVPG without adverse events [83, 84]. Following these successful results, the same group published the first human pilot study, including 28 patients with chronic liver disease, where portal pressure gradient results were compared with clinical signs of PH. The obtained results correlated well with clinical parameters of PH (presence of varices, PH gastropathy, and thrombocytopenia), and the technical success rate was 100% [85]. The direct correlation between portal pressure measurement by EUS and HVPG in humans was studied by Zhang et al. [86], and similar results were found by both techniques without a significant difference in time needed to perform or adverse events. EUS may also have a role for non-invasive evaluation of liver fibrosis, not only in patients that failed with non-invasive techniques (due to obesity, ascites, thick sub-cutaneous fat, or restricted intercostal spaces) but also has the advantage of performing shear-wave elastography of both the right and left liver in the same procedure [77, 87]. A study by Robles-Medranda et al. [88] found a similar accuracy of EUS-elastography when compared to liver and spleen stiffness for the prediction of PH. In pooled analysis, EUS liver and spleen elastography parameters predicted liver cirrhosis and PH with high sensitivities and negative predictive values. However, there are limitations such as technical difficulties (lack of experienced endosonographers), high costs, need for sedation, and, although low, the expected risks associated with the procedure (mainly bleeding, perforation, and infectious complications) [81, 83].
Conclusion
The development of PH in a patient with cirrhosis is a pivotal event that profoundly impacts the prognosis and management. While HVPG measurement is considered the gold standard for evaluating PH, it is invasive and not an easily available procedure.
Serum biomarkers and scoring systems have been ex-plored to predict the presence of PH. However, their individual performance has been suboptimal. Combining multiple markers or scores can enhance diagnostic accuracy. Echoendoscopy-guided shear-wave evaluation of the liver and spleen might be an interesting option in patients who failed transient elastography, as it also has the possibility for screening for varices, liver biopsy, or portal pressure gradient measurement.
The Baveno VII guidelines emphasize the significance of non-invasive evaluation in cirrhotic patients and recommend the use of liver and SSMs for early identification of individuals at risk of developing CSPH. This proactive approach enables the detection of patients with compensated cirrhosis/chronic advanced chronic liver disease with CSPH and the initiation of appropriate treatment to reduce the risk of decompensation of cirrhosis and improve patient outcomes.