Case Report
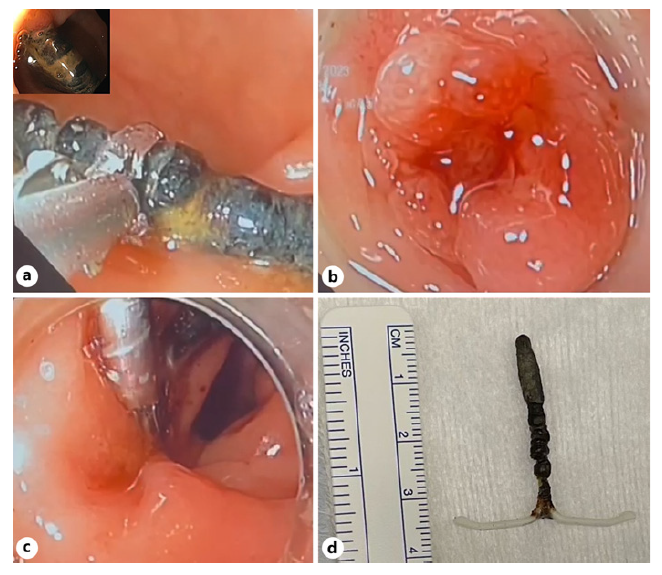
A 58-year-old asymptomatic woman was referred to the gynaecology clinic due to an incidentally found intrauterine device (IUD) in a pelvic X-ray. The patient inserted the device 15 years ago, but it was thought to have been naturally expelled 1 year later during an unplanned pregnancy. Since it was implanted at a different institution, no information about the type of IUD was available. A subsequent pelvic and abdominal CT scan revealed the migration of the IUD to the rectum, confirmed by a sigmoidoscopy that demonstrated its transmural placement in a partially intraluminal position in the rectum, at 12 cm from the anal verge (Fig. 1, 2). After a multidisciplinary discussion with gastroenterologists, gynaecologists, and surgeons, it was decided to attempt an endoscopic removal with salvage surgical intervention if deemed necessary. The procedure was performed in the operating room under conscious sedation after bowel preparation and the device was removed in one piece using grasping forceps traction. The defect was prophylactically closed with three through-the-scope clips. The patient underwent 24-hour hospital surveillance with no immediate post-procedure complications. Prophylactic antibiotic therapy with amoxicillinclavulanic acid was initiated and continued for 1 week. After 1 month of clinical follow-up, the patient remained asymptomatic and free of further complications.
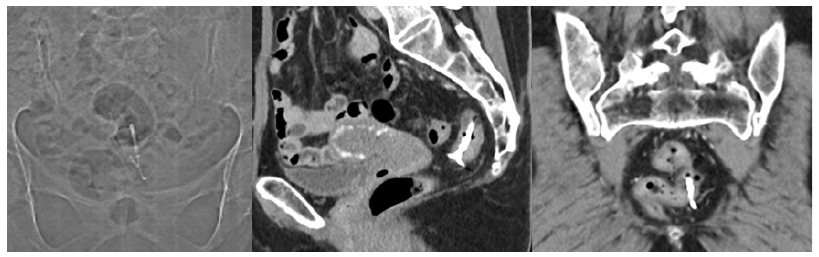
Fig. 1 CT scan revealing the location of the intrauterine device with one stalk in the lumen of the rectum and the rest of the device in the mesorectum, unrelated to the uterus, below the peritoneal reflection.
Discussion
IUDs are commonly employed for birth control but can lead to severe complications such as uterine perforation and intra-abdominal migration (0.06-0.16%), including adjacent organ involvement, such as the digestive tract [1]. The sigmoid colon is the most common site of intestinal perforation, with the rectum affected in 1/5 of the cases [2]. Approximately 15 cases of rectal IUD migration have been reported in the literature [2]. Migration can occur during insertion or gradually over time. Risk factors for perforation include IUD type, operator skill, uterus size/position, postpartum insertion within 6 months, lactation (due to low estrogen levels leading to uterine atrophy), and unplanned pregnancy with an inserted IUD, as observed in our case [3]. Patients may experience lower abdominal pain, fever, rectal bleeding and/or diarrhoea, but many remain asymptomatic until the migrating device is incidentally detected. In some cases, IUD strings protrude from the rectum [3]. Regardless of presentation, migrated IUDs always require removal due to potential complications, including infection, bowel perforation, obstruction, fistula formation, and abscesses [4].
Removal of migrated IUDs can be made endoscopically, surgically or using a combined approach [1, 2, 5]. The success of endoscopic removal depends on the location of the device and depth of the perforation. Endoscopic removal is effective for recto-sigmoid migration without adjacent organ involvement or complications. Following IUD extraction, closure of the defect typically involves the use of through-the-scope or over-the-scope clips [2, 5]. The selection between these devices primarily hinges on the specific location of the defect.
This report highlights the successful endoscopic removal of a rare rectally perforated IUD. It underscores the importance of tailored decision-making and vigilant monitoring for IUD migration, especially in patients who become pregnant after IUD insertion.