Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Revista Portuguesa de Imunoalergologia
versão impressa ISSN 0871-9721
Rev Port Imunoalergologia vol.28 no.4 Lisboa dez. 2020
https://doi.org/10.32932/rpia.2020.12.046
ARTIGO ORIGINAL
Identification of clusters of asthma control: A preliminary analysis of the Inspirers studies
Identificar clusters de controlo da asma: Uma análise preliminar dos estudos Inspirers
Cristina Jácome1, Rita Amaral1,2, Rute Almeida1, Ana Margarida Pereira3, Mariana Couto4, Luís Araújo3, Magna Alves-Correia3, Mariana Pereira1,5, Manuel Ferreira-Magalhães1,6, Cláudia Chaves Loureiro7, Maria Joana Catarata7, Lília Maia Santos7, Sara Cabral7, João Pereira7, Bárbara Ramos7, Cristina Lopes8,9, Ana Mendes10, Anabela Lopes10, José Carlos Cidrais Rodrigues11, Georgeta Oliveira11, Ana Paula Aguiar11, Ivete Afonso11, Joana Carvalho11, Ana Maria Arrobas7, José Costa7, Margarida Valério7, Marta Pereira7, Teresa Almeida7, Joana Dias7, Ana Todo Bom12, João Azevedo13, Carmelita Ribeiro12, Marta Alves12, Paula Leiria Pinto14, Nuno Neuparth14,15, Ana Castro Neves14, Ana Palhinha14, João Gaspar Marques14,15, Pedro Martins14,15, David Pina Trincão16, Filipa Todo Bom17, Maria Alvarenga Santos17, Joana Branco17, Alberto Costa18, Armandina Silva Neto18, Marta Santalha18, Carlos Lozoya19, Natacha Santos20, Diana Silva21, Maria João Vasconcelos21, Luís Taborda Barata22, Maria Fernanda Teixeira6, Diana Pinto6, Rodrigo Rodrigues Alves23, Ana Sofia Moreira23, Cláudia Sofia Pinto24, Pedro Morais Silva25, Carlos Alves26, Raquel Câmara26, Didina Coelho26, Diana Bordalo1,27, Fernanda Carvalho27, Ricardo Fernandes28,29, Rosário Ferreira28, José Ferraz de Oliveira30, Fernando Menezes31, Ricardo Gomes31, Maria José Calix32, Ana Marques32, João Cardoso33, Madalena Emiliano33, Rita Gerardo33, Carlos Nunes34, José Alberto Ferreira35, Inês Lopes35, Adelaide Alves36, João Almeida Fonseca*1,3,5,37, INSPIRERS group
1 CINTESIS – Center for Health Technology and Services Research, Faculty of Medicine, University of Porto
2 Dept. of Cardiovascular and Respiratory Sciences, Porto Health School, Polytechnic Institute of Porto
3 Imunoalergologia, CUF Porto Instituto & Hospital
4 Imunoalergologia, José de Mello Saúde
5 MEDIDA – Medicina, Educação, Investigação, Desenvolvimento e Avaliação, Porto
6 Serviço de Pediatria, Centro Materno Infantil do Norte, Centro Hospitalar Universitário do Porto
7 Serviço de Pneumologia, Centro Hospitalar e Universitário de Coimbra
8 Unidade de Imunoalergologia, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos
9 Imunologia Básica e Clínica, Faculdade de Medicina, Universidade do Porto
10 Serviço de Imunoalergologia, Hospital de Santa Maria, Centro Hospitalar Lisboa Norte
11 Serviço de Pediatria, Hospital Pedro Hispano, Unidade Local de Saúde de Matosinhos
12 Serviço de Imunoalergologia, Centro Hospitalar e Universitário de Coimbra
13 Imunoalergologia, Centro Hospitalar de Leiria
14 Serviço de Imunoalergologia, Hospital de Dona Estefânia, Centro Hospitalar Universitário de Lisboa Central
15 Pathophysiology, CHRC, Environmental Health research group, CEDOC, NOVA Medical School / Faculdade de Ciências Médicas, Lisboa
16 Serviço de Imunoalergologia, Hospital Rainha Santa Isabel, Centro Hospitalar do Médio Tejo, Torres Novas
17 Serviço de Pneumologia, Hospital Beatriz Ângelo, Loures
18 Serviço de Pediatria, Hospital da Senhora da Oliveira, Guimarães
19 Serviço de Imunoalergologia, Hospital Amato Lusitano, Unidade Local de Saúde de Castelo Branco
20 Serviço de Imunoalergologia, Centro Hospitalar Universitário do Algarve, Portimão
21 Serviço de Imunoalergologia, Centro Hospitalar de São João, Porto
22 Serviço de Imunoalergologia, Hospital Pêro da Covilhã, Centro Hospitalar Universitário Cova da Beira, Covilhã
23 Unidade de Imunoalergologia, Hospital do Divino Espirito Santo, Ponta Delgada
24 Serviço de Pneumologia, Hospital São Pedro de Vila Real, Centro Hospitalar de Trás‑os‑Montes e Alto Douro, Vila Real
25 Imunoalergologia, Grupo HPA Saúde, Portimão
26 Serviço de Pneumologia, Hospital Nossa Senhora do Rosário, Centro Hospitalar Barreiro Montijo, Barreiro
27 Serviço de Pediatria, Unidade Hospitalar de Famalicão, Centro Hospitalar do Médio Ave, Vila Nova de Famalicão
28 Serviço de Pediatria, Departamento de Pediatria, Hospital de Santa Maria, Centro Hospitalar de Lisboa Norte
29 Laboratório de Farmacologia Clínica e Terapêutica, Instituto de Medicina Molecular, Faculdade de Medicina, Universidade de Lisboa
30 Imunoalergologia, Hospital Privado de Alfena, Trofa Saúde, Alfena
31 Serviço de Pneumologia, Hospital Garcia de Orta, Almada
32 Serviço de Pediatria, Hospital de São Teotónio, Centro Hospitalar Tondela-Viseu
33 Serviço de Pneumologia, Hospital Santa Marta, Centro Hospitalar de Lisboa Central, Lisboa
34 Imunoalergologia, Centro de Imunoalergologia do Algarve, Portimão
35 Serviço de Imunoalergologia, Unidade I, Centro Hospitalar Vila Nova de Gaia/Espinho
36 Serviço de Pneumologia, Unidade I, Centro Hospitalar Vila Nova de Gaia/Espinho
37 MEDCIDS – Departamento de Medicina da Comunidade Informação e Decisão em Saúde, Faculdade de Medicina, Universidade do Porto
ABSTRACT
Aims: To identify distinct asthma control clusters based on Control of Allergic Rhinitis and Asthma Test (CARAT) and to compare patients characteristics among these clusters. Methods: Adults and adolescents (≥13 years) with persistent asthma were recruited at 29 Portuguese hospital outpatient clinics, in the context of two observational studies of the INSPIRERS project. Demographic and clinical characteristics, adherence to inhaled medication, beliefs about inhaled medication, anxiety and depression, quality of life, and asthma control (CARAT, >24 good control) were collected. Hierarchical cluster analysis was performed using CARAT total score (CARAT‑T). Results: 410 patients (68% adults), with a median (percentile 25–percentile 75) age of 28 (16‑46) years, were analysed. Three clusters were identified [mean CARAT‑T (min‑max)]: cluster 1 [27(24‑30)], cluster 2 [19(14‑23)] and cluster 3 [10(2‑13)]. Patients in cluster 1 (34%) were characterised by better asthma control, better quality of life, higher inhaler adherence and use of a single inhaler. Patients in clusters 2 (50%) and 3 (16%) had uncontrolled asthma, lower inhaler adherence, more symptoms of anxiety and depression and more than half had at least one exacerbation in the previous year. Furthermore, patients in cluster 3 were predominantly female, had more unscheduled medical visits and more anxiety symptoms, perceived a higher necessity of their prescribed inhalers but also higher levels of concern about taking these inhalers. There were no differences in age, body mass index, lung function, smoking status, hospital admissions or specialist physician follow‑up time among the three clusters. Conclusion: An unsupervised method based on CARAT‑T, identified 3 clusters of patients with distinct, clinically meaningful characteristics. The cluster with better asthma control had a cut‑off similar to the established in the validation study of CARAT and an additional cut‑off seems to distinguish more severe disease. Further research is necessary to validate the asthma control clusters identified.
Keywords: Asthma, asthma control, classification, cluster analysis, control of allergic rhinitis and asthma test.
RESUMO
Objetivos: Identificar clusters de controlo da asma baseados no Teste de Controlo da Asma e Rinite Alérgica (CARAT) e comparar as características dos doentes nos diferentes clusters. Métodos: Adultos e adolescentes (≥13 anos) com asma persistente foram recrutados em 29 centros no contexto de 2 estudos observacionais do projeto INSPIRERS. Foram recolhidos dados relativos a características demográficas e clínicas, adesão ao inalador, crenças sobre a medicação, sintomas de ansiedade e depressão, qualidade de vida e controlo da asma (CARAT, >24 bom controlo). Foi efetuada uma análise hierárquica de clusters usando a pontuação total do CARAT (CARAT‑T).Resultados: Foram analisados 410 doentes (68% adultos), com uma idade mediana (percentil 25–percentil 75) de 28 (16‑46) anos. Foram identificados três clusters [média CARAT‑T(min‑max)]: cluster 1 [27(24‑30)], cluster 2 [19(14‑23)] e cluster 3 [10(2‑13)]. Os doentes no cluster 1 (34%) apresentavam melhor controlo da asma, melhor qualidade de vida, maior adesão aos inaladores e usavam um único inalador. Os doentes nos clusters 2 (50%) e 3 (16%) tinham a asma não controlada, menor adesão aos inaladores, mais sintomas de ansiedade e depressão e mais de metade reportavam pelo menos uma exacerbação no último ano. Adicionalmente, os doentes no cluster 3 eram predominantemente mulheres, tinham mais consultas médicas não agendadas, apresentavam mais sintomas de ansiedade, percebiam uma maior necessidade dos inaladores, mas também uma maior preocupação associada ao seu uso. Não se verificaram diferenças na idade, índice de massa corporal, função pulmonar, hábitos tabágicos, hospitalizações ou tempo de seguimento pelo médico especialista. Conclusões: Um método não supervisionado baseado no CARAT‑T, identificou 3 clusters de doentes com diferentes características clínicas. O cluster com melhor controlo apresenta um ponto de corte semelhante ao estabelecido no estudo de validação do CARAT. Este estudo sugere ainda a existência de um ponto de corte adicional para distinguir doença mais grave. Mais investigação é necessária para validar os clusters identificados.
Palavras‑chave:
Análise de clusters, asma, classificação, controlo da asma, teste de controlo da asma e rinite alérgica.
INTRODUCTION
Asthma is a common, chronic disease characterized by airway inflammation1. The long‑term goals of asthma management are to achieve good control of symptoms and minimise future risk of exacerbations1. However, large observational studies have shown that these goals are not being easily achieved, even in patients with mild disease2‑4.
Treatments can be very successful for some patients but not for others, leading to poor outcomes, unnecessary suffering and elevated direct and indirect costs5,6.
This is mainly related to the heterogeneous nature of asthma1, supporting the existence of distinct asthma control profiles, which differ in their characteristics and treatment responsiveness.
Unsupervised methods, particularly cluster analysis, have been used to identify subtypes of asthma with similar characteristics to predict future risks and/or to contribute for the implementation of personalised therapies7,8.
However, most of the studies that identified asthma clusters were based on clinical and inflammatory biomarkers9‑12 of adult patients with moderate to severe asthma, preventing clusters generalization to younger and/or less severe patients and their implementation into real practice. Further studies that explore subtypes of asthma control using data easily obtained in clinical practice, such as questionnaire‑based information, could unravel the complex links underlying asthma control.
The Control of Allergic Rhinitis and Asthma Test (CARAT) is a self‑administered questionnaire commonly used to assess asthma control both in clinical studies and clinical practice13. It is composed of 10 questions that address upper and lower airway symptoms, sleep interference, activities limitation, and the need to increase medication over a 4‑week period. The total score (CARAT‑T) is calculated by summing the score of each question, resulting in a range of 0–30 points. In the original validation study, a CARAT‑T>24 was indicative of good disease control. This work also showed that CARAT‑T can be used to compare groups and to evaluate individual patients over time. However, to date, the CARAT‑T ability to identify asthma control clusters was not explored.
This preliminary study aimed to identify asthma control clusters based on CARAT‑T in a cohort of Portuguese adolescents and adults with persistent asthma and to compare patients characteristics among the obtained clusters.
MATERIAL AND METHODS
Study design
Two prospective observational studies of the INSPIRERS project were analysed14,15 and data from initial face‑to‑face visit and 1‑week telephone interview were collected.
A convenience sample of patients with persistent asthma was recruited between November 2017 and June 2018 at 29 allergy, pulmonology and paediatric secondary care outpatient clinics covering 6 Portugal regions (North, Center, Lisbon, Algarve, Azores, Madeira). The study protocols were approved by the ethics committees of all participating centres and by the national data protection committee. The study was conducted in accordance with the ethical standards established in the Declaration of Helsinki of 1946. Eligible patients were approached by physicians during medical visits and written informed consent was obtained before enrolment in the study.
Adult patients signed a consent form; adolescents signed an assent form and a parental consent form was also obtained. The study is reported according to STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines16.
Participants
Patients were included if they had a previous medical diagnosis of persistent asthma; were at least 13 years old (13‑17 years adolescents; ≥18 years adults); and had an active prescription for an inhaled controller medication for asthma. All inhaled controller treatments were allowed and there was no change in any prescribed medication in relation to the participation in these studies. Patients were excluded if they had a diagnosis of a chronic lung disease other than asthma or a diagnosis of another significant chronic condition with possible interference with the study aims.
Data collection
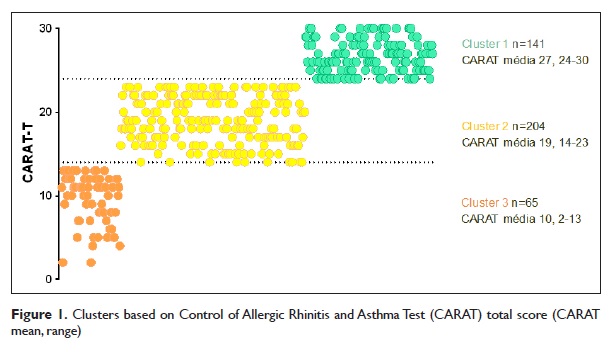
During the initial face‑to‑face visit, data were collected from both physicians and patients. Physicians answered a questionnaire including: assessment of patients asthma control according to the Global Initiative for Asthma (GINA)1; last known value of percent predicted Forced Expiratory Volume in first second (FEV1); number of exacerbations, defined as episodes of progressive increase in shortness of breath, cough, wheezing, and/or chest tightness, requiring change in maintenance therapy17; use of health care resources, namely number of unscheduled medical visits and number of hospital admissions; and specialist follow‑up time (measured in months). Physicians also reported the patients current asthma treatment, including inhaled medication, allergen immunotherapy and biologic therapy.
Demographic – age, gender, smoking habits – and anthropometric – height, weight – data were collected from patients, along with health information as described next. Asthma control in the patients perspective was assessed with CARAT13. The total score (CARAT‑T) is calculated by summing the score of each of the 10 questions, resulting in a range of 0–30 points. A score >24 indicates good disease control.
The Portuguese version of the Hospital Anxiety and Depression Scale (HADS) was used to assess anxiety and depression symptoms18. The HADS contains 14 items, seven measuring anxiety symptoms (HADS‑A) and seven depression symptoms (HADS‑D), which are scored separately.
Each item has a 4‑point response category, so the possible scores range from 0 (minimum symptom load) to 21 (maximum symptom load) for HADS‑A and for HADS‑D.
A score ≥8 in the HADS‑A/HADS‑D was used to consider the presence of clinically significant anxiety and depression symptoms18.
EQ‑5D visual analogue scale (VAS) was used to assess health‑related quality of life19,20. The VAS ranges from 0 (worst imaginable health state) to 100 (best imaginable health state) millimetres. Patients assessed their global adherence to inhaled controller medication for asthma during the previous week also using a VAS, ranging from 0 (worst) to 100 (best) millimetres21.
Approximately one week later14, through a telephone interview, patients were asked about their attitudes and beliefs in relation to their inhalers using the Portuguese version of the specific Beliefs about Medicines Questionnaire (BMQ‑Specific)22. The BMQ‑Specific is an 11‑item questionnaire that comprises two subscales: a 5‑item Necessity scale, to assess beliefs about the necessity for Prescribed medication (Specific‑Necessity), and a 6‑item Concerns scale, to assess beliefs about the danger of dependence and long‑term toxicity and the disruptive effects of medication (Specific‑Concerns).
Each item is scored on a 5‑point Likert scale (from 1 = strongly disagree to 5 = strongly agree), and the total scores for the Necessity and Concerns subscales range from 5 to 25 and from 6 to 30, respectively. The higher the score, the greater is the patients belief in the concept represented by the scale22.
Statistical Analysis
Descriptive statistics were used to characterise the sample. Normality of each variable was investigated with Kolmogorov‑Smirnov Tests and visual analysis of histograms. To identify asthma control profiles based on CARAT‑T, we conducted hierarchical cluster analysis using a squared Euclidean distance measure to assess similarity/dissimilarity across variables, and between‑groups linkage method for combining clusters. The Bayesian Information Criterion (BIC) was also calculated for the number of clusters obtained. To compare differences among clusters for continuous variables, one‑way ANOVA (normally distributed) or Kruskal‑Wallis test (not normally distributed) with Bonferroni correction were used. Chi‑square tests were used for categorical variables. Statistical analyses were performed using IBM SPSS Statistics version 25.0 (IBM Corporation, Armonk, NY, USA) and plots were created using GraphPad Prism version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). The level of significance was set at 0.05.
RESULTS
Participants
From the 413 patients included in both studies, 410 had complete data on CARAT. Patients were mostly adults (68%) and female (61%). Characteristics of the participants are shown in Table 1.
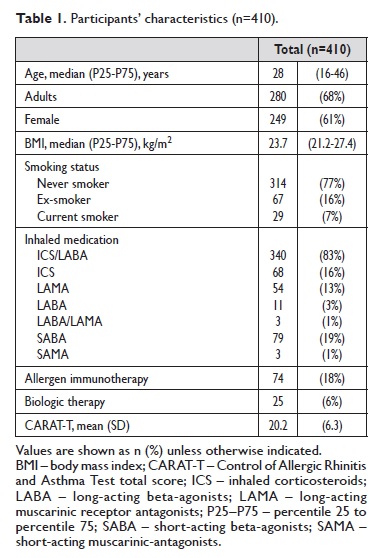
Clusters of asthma control
Three clusters were identified as shown in Figure 1 (BIC= 97.5). Cluster 2 was the largest cluster including half of the participants (n=204, 50%), followed by cluster 1 (n=141, 34%) and cluster 3 (n=65, 16%). Characteristics of each cluster are presented on Table 2. Most patients in cluster 1 had their asthma controlled both using the CARAT cut‑off or GINA classification.
They were also characterized by higher inhaler adherence, use of a single inhaler (45% in a once‑daily regimen), better quality of life and equal gender distribution.
All patients in clusters 2 and 3 had uncontrolled asthma using the CARAT cut‑off and more than half had partly controlled/uncontrolled asthma using GINA classification.
Patients in clusters 2 and 3 had also lower inhaler adherence, more than half had at least one exacerbation in the previous year and more symptoms of anxiety and depression.
Furthermore, patients in cluster 3 were predominantly female, had more unscheduled medical visits, presented more symptoms of anxiety, perceived a higher necessity of their prescribed inhalers but also higher levels of concern about taking these inhalers. There were no significant differences in age, BMI, FEV1, smoking status, hospital admissions or specialist follow‑up time among clusters.
DISCUSSION
To our knowledge, this is the first study using an unsupervised method to identify subgroups of asthma control solely based on the total score of the self‑reported questionnaire CARAT. We were able to identify 3 clusters of patients that differed in a number of characteristics such as adherence to inhaled medication, beliefs about inhaled medication, number of inhalers prescribed, number of exacerbations and unscheduled medical visits in the previous year, symptoms of anxiety and depression, health‑related quality of life and gender distribution. The clusters found were not determined by any a priori clinical knowledge, but rather on the total score of an asthma control self‑reported questionnaire, easily applied in daily clinical practice. From the distributions of CARAT‑T across the 3 clusters, it is possible to observe that two cut‑offs (≥24 and ≥14) emerged from the unsupervised analyses. The cut‑off for controlled disease is in line with the cut‑off suggested in the original validation study of CARAT (>24)23. The slight difference might be related with differences in the samples used. The validation study included a smaller sample of adults with asthma (n=62) recruited from 4 allergy secondary care outpatient clinics in three Portuguese regions23; while in the present study a larger and more representative sample was used: adolescents were also included and patients were recruited by 3 medical specialities (allergy, pulmonology and paediatrics) from 6 Portuguese regions. An additional CARAT cut‑off of 14 is for the first time proposed and seems to be able to distinguish patients with partly controlled disease and uncontrolled disease. This cut‑off may be useful to clinicians to identify individuals with different risk levels and to individualize care in order to maximise outcomes.
Nevertheless, this cut‑off needs further validation before implementation into clinical practice.
Patients in the cluster with better asthma control had higher self‑reported adherence to inhaled medication compared to patients from the other two clusters. Although it is possible that all patients were overestimating real adherence24,25, this result suggests that asthma control is associated with adherence behaviours. In addition, most patients in this cluster had a single inhaler, and almost half in a once‑daily regimen; possibly suggesting that simple therapeutic plans are related with better adherence and asthma control26. A randomized control study with patients with asthma has previously shown that using a single inhaler for both maintenance treatment and symptom relief increased the dose of inhaled corticosteroid taken27. But evidence on the link between adherence and number of drugs or daily doses is still inconclusive28.
This may be related to the fact that there is no “gold standard” in evaluating adherence29. Patients in the cluster with worst asthma control perceived a higher necessity of their prescribed inhalers, but also higher levels of concern about taking these inhalers. Indeed, more concerns about medication have been related to worse adherence28.
Patients from clusters 2 and 3, as expected, had more exacerbations than patients from cluster 1, as result of poor disease control. These patients had also poorer quality of life and more frequently depression. These characteristics have been previously found to distinguish asthma subtypes7,10,11. Patients in cluster 3 were in addition predominantly female, had more unscheduled medical visits, presented more symptoms of anxiety. These characteristics are commonly present in patients with difficult to treat asthma or severe asthma30. In fact, this cluster includes 16% of the patients, which is in line with described prevalence of difficult‑to‑treat asthma (17%)31 and severe asthma (3‑10%)31,32. Strengths and limitations of this study should be recognized.
The inclusion of adolescents with persistent asthma in our sample and the recruitment at 29 secondary healthcare centres are strengths of the present study. Yet patients were recruited by convenience sampling, which limits the generalizability of the results. Population studies with larger sample size and an extended age range (including children and older adults) and from other healthcare settings (e.g. primary care) should be conducted in future.
Another strength of our study was the use of variables easily obtained in clinical practice. Patients allergic profile, treatment step and comorbidities (e.g., rhinitis) were not analysed but might also play a role in cluster differentiation.
Future studies could address these issues in order to validate the three asthma control clusters identified or identify other types. These asthma control clusters were identified using a single clustering method – hierarchical cluster analysis and a single variable – CARAT‑T. Although this is one of the most common clustering methods used in asthma8,10, future studies using CARAT‑T and/or other self‑reported variables could also use other unsupervised methods that shown to be promising in identifying clusters of subjects with allergic respiratory diseases, such as latent class or profile analysis33,34.
CONCLUSIONS
Using an unsupervised method based on CARAT‑T, we identified 3 clusters of patients with distinct, clinically meaningful characteristics. The cluster with better asthma control generated by this unsupervised method had a cut‑value similar to the established in the validation study of CARAT. An additional cut‑off, that seems to distinguish more severe disease also emerged from this preliminary analysis. Further research is necessary to better characterize the identified clusters of asthma control.
REFERENCES
1. Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention, 2019. Available from: www.ginasthma.org. [ Links ]
2. Postma DS, Brightling C, Baldi S, Van den Berge M, Fabbri LM, Gagnatelli A, et al. Exploring the relevance and extent of small airways dysfunction in asthma (ATLANTIS): baseline data from a prospective cohort study. The Lancet Respir Med 2019;7(5):402‑16. [ Links ]
3. Backman H, Jansson S‑A, Stridsman C, Muellerova H, Wurst K, Hedman L, et al. Chronic airway obstruction in a population‑based adult asthma cohort: Prevalence, incidence and prognostic factors. Respir Med 2018;138:115‑22. [ Links ]
4. Price D, Fletcher M, van der Molen T. Asthma control and management in 8,000 European patients: the REcognise Asthma and LInk to Symptoms and Experience (REALISE) survey. NPJ Prim Care Respir Med 2014;24:14009. [ Links ]
5. Demoly P, Annunziata K, Gubba E, Adamek L. Repeated cross‑sectional survey of patient‑reported asthma control in Europe in the past 5 years. Eur Respir Rev 2012;21(123):66‑74. [ Links ]
6. Barbosa JP, Ferreira‑Magalhaes M, Sa‑Sousa A, Azevedo LF, Fonseca JA. Cost of asthma in Portuguese adults: A population‑based, cost‑of‑illness study. Rev Port Pneumol (2006) 2017;23(6):323‑30. [ Links ]
7. Baptist AP, Hao W, Karamched KR, Kaur B, Carpenter L, Song PXK. Distinct Asthma Phenotypes Among Older Adults with Asthma. J Allergy Clin Immunol Pract 2018;6(1):244‑9.e2. [ Links ]
8. Delgado‑Eckert E, Fuchs O, Kumar N, Pekkanen J, Dalphin JC, Riedler J, et al. Functional phenotypes determined by fluctuation‑based clustering of lung function measurements in healthy and asthmatic cohort participants. Thorax 2018;73(2):107‑15. [ Links ]
9. Lefaudeux D, De Meulder B, Loza MJ, Peffer N, Rowe A, Baribaud F, et al. U‑BIOPRED clinical adult asthma clusters linked to a subset of sputum omics. J Allergy Clin Immunol 2017;139(6):1797‑807. [ Links ]
10. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. Identification of asthma phenotypes using cluster analysis inthe Severe Asthma Research Program. Am J Respir Crit CareMed 2010;181(4):315‑23. [ Links ]
11. Wu W, Bleecker E, Moore W, Busse WW, Castro M, Chung KF, et al. Unsupervised phenotyping of Severe Asthma Research Program participants using expanded lung data. J Allergy Clin Immunol 2014;133(5):1280‑8. [ Links ]
12. van der Molen T, Fletcher M, Price D. Identifying Patient Attitudinal Clusters Associated with Asthma Control: The European REALISE Survey. J Allergy Clin Immunol Pract 2018;6(3):962‑71. [ Links ]
13. Fonseca JA, Nogueira‑Silva L, Morais‑Almeida M, Azevedo L, Sa‑Sousa
A, Branco‑Ferreira M, et al. Validation of a questionnaire (CARAT10) to assess rhinitis and asthma in patients with asthma. Allergy 2010;65(8):1042‑8.
14. Jácome C, Guedes R, Almeida R, Teixeira JF, Pinho B, Vieira‑Marques P, et al. mINSPIRERS – Estudo da exequibilidade de uma aplicação móvel para medição e melhoria da adesão à medicação inalada de controlo em adolescentes e adultos com asma persistente. Protocolo de um estudo observacional multicêntrico. Ver Port Imunoalergologia 2018;26:47‑61.
15. Jacome C, Pereira AM, Almeida R, Ferreira‑Magalhaes M, Couto M, Araujo L, et al Patient‑physician discordance in assessment of adherence to inhaled controller medication: a cross‑sectional analysis of two cohorts. BMJ Open 2019;9(11):e031732.
16. Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg 2014;12(12):1500‑24. [ Links ]
17. Loymans RJ, Ter Riet G, Sterk PJ. Definitions of asthma exacerbations. Curr Opin Allergy Clin Immunol 2011;11(3):181‑6. [ Links ]
18. Pais‑Ribeiro J, Silva I, Ferreira T, Martins A, Meneses R, Baltar M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol Health Med 2007;12(2):225‑35; quiz 35‑7. [ Links ]
19. Hung MC, Lu WS, Chen SS, Hou WH, Hsieh CL, Wang JD. Validation of the EQ‑5D in Patients with Traumatic Limb Injury. J Occup Rehabil 2015;25(2):387‑93. [ Links ]
20. McPhail S, Lane P, Russell T, Brauer SG, Urry S, Jasiewicz J, et al. Telephone reliability of the Frenchay Activity Index and EQ‑5D amongst older adults. Health Qual Life Outcomes 2009;7(1):48. [ Links ]
21. Jonsdottir H, Opjordsmoen S, Birkenaes AB, Engh JA, Ringen PA, Vaskinn A, et al. Medication adherence in outpatients with severe mental disorders: relation between self‑reports and serum level. J Clin Psychopharmacol 2010;30(2):169‑75. [ Links ]
22. Salgado T, Marques A, Geraldes L, Benrimoj S, Horne R, Fernandez‑Llimos F. Cross‑cultural adaptation of The Beliefs about Medicines Questionnaire into Portuguese. Sao Paulo medical journal = Revista paulista de medicina 2013;131(2):88‑94.
23. Fonseca JA, Nogueira‑Silva L, Morais‑Almeida M, Sa‑Sousa A, Azevedo LF, Ferreira J, et al. Control of Allergic Rhinitis and Asthma Test (CARAT) can be used to assess individual patients over time. Clin Transl Allergy 2012;2(1):16. [ Links ]
24. Nau DP, Steinke DT, Williams LK, Austin R, Lafata JE, Divine G, et al. Adherence analysis using visual analog scale versus claims‑based estimation. Ann Pharmacother 2007;41(11):1792‑7. [ Links ]
25. Kalichman SC, Amaral CM, Swetzes C, Jones M, Macy R, Kalichman MO, et al. A simple single‑item rating scale to measure medication adherence: further evidence for convergent validity. J Int Assoc Physicians AIDS Care (Chic) 2009;8(6):367‑74. [ Links ]
26. Hassan M, Davies SE, Trethewey SP, Mansur AH. Prevalence and predictors of adherence to controller therapy in adult patients with severe/difficult‑to‑treat asthma: a systematic review and meta‑analysis. J Asthma 2019;in press:1‑10. [ Links ]
27. Sovani MP, Whale CI, Oborne J, Cooper S, Mortimer K, Ekstrom T, et al. Poor adherence with inhaled corticosteroids for asthma: can using a single inhaler containing budesonide and formoterol help? Br J Gen Pract 2008;58(546):37‑43. [ Links ]
28. Dima AL, Hernandez G, Cunillera O, Ferrer M, de Bruin M. Asthma inhaler adherence determinants in adults: systematic review of observational data. Eur Respir J 2015;45(4):994‑1018. [ Links ]
29. Anghel LA, Farcas AM, Oprean RN. An overview of the commonmethods used to measure treatment adherence. Med Pharm Rep 2019;92(2):117‑22. [ Links ]
30. Global Initiative for Asthma. Difficult‑to‑treat & Severe Asthma in adolescents and adult patients: Diagnosis and Management, 2019. Available from:www.ginasthma.org. [ Links ]
31. Hekking P‑PW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol 2015;135(4):896‑902. [ Links ]
32. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation andtreatment of severe asthma. Eur Respir J 2014;43(2):343. [ Links ]
33. Amaral R, Bousquet J, Pereira AM, Araújo LM, Sa‑Sousa A, Jacinto T, et al Disentangling the heterogeneity of allergic respiratory diseases by latent class analysis reveals novel phenotypes. Allergy 2019;74(4):698‑708. [ Links ]
34. Siroux V, Basagaña X, Boudier A, Pin I, Garcia‑Aymerich J, Vesin A, et al. Identifying adult asthma phenotypes using a clustering approach. Eur Respir J 2011;38(2):310. [ Links ]
João Almeida Fonseca, MD, PhD
Center for Health Technology and Services Research (CINTESIS)
Faculty of Medicine, Universidade do Porto
Rua Dr. Plácido da Costa, Porto 4200-450, Portugal
E-mail: fonseca.ja@gmail.com
Acknowledgments
We thank the participants and centres involved in the project Inspirers.
Funding
This work was funded by ERDF (European Regional Development Fund) through the operations: POCI-01-0145-FEDER-029130 (“mINSPIRERS–mHealth to measure and improve adherence to medication in chronic obstructive respiratory diseases - generalisation and evaluation of gamification, peer support and advanced image processing technologies”) co-funded by the COMPETE2020 (Programa Operacional Competitividade e Internacionalização), Portugal 2020 and by Portuguese Funds through FCT (Fundação para a Ciência e a Tecnologia).
Conflicts of interest
None declared.
Data de receção / Received in: 16/12/2019
Data de aceitação / Accepted for publication in: 20/01/2020














