INTRODUCTION
Chronic respiratory diseases, such as asthma, are a source of substantial burden, with increased morbidity and mortality1. Among patients with asthma, those with severe asthma have disproportionately higher morbidity, mortality, and costs than patients with non-severe asthma, representing an unmet clinical need 2. In Portugal, the first and only Portuguese National Asthma Survey (INAsma) estimated that the prevalence of asthma in the Portuguese population was 6.8% (95%CI 6.0-7.7)3. Almost half (43%) of the patients had non-controlled asthma, which is associated with worse health-related quality of life 4. Adequate self-management dramatically reduces asthma morbidity, including reduced hospitalisations, emergency department visits, unscheduled medical visits, nocturnal awakening and absenteeism 5.
Digital technologies for asthma are increasing and can effectively engage patients in the self-management of their disease, reduce exacerbations and improve patients’ quality of life 6-8. Still, most asthma apps do not attract and retain users 9. Indeed, although three-quarters of patients with asthma had downloaded/ used a general app, only one-third had ever used a health and fitness app, and 3% an asthma app 10. This reality may be attributed to various factors, but app development being detached from the scientific evidence and lack of patients’ meaningful involvement are among the most relevant 11.
Digital health will only reach its full potential when apps for patients are aligned with their perceived needs and are fully integrated into the clinical practice 12.
Therefore, a co-design development process with users in iterative cycles is recommended to align the concerns of both patients and healthcare professionals 13.
Patient and Public Involvement (PPI) in research refers to “research being carried out ‘with’ or ‘by’ members of the public (including patients, potential patients, carers and people who use health and social care services) rather than ‘to’, ‘about’ or ‘for’ them”14. Meaningful PPI in digital health improved successful implementation, usability, insight into patients’ needs and preferences, high satisfaction levels, credibility and the likelihood of app recommendation use 11. However, patients are mostly involved in the final stages of digital solutions development, often in usability tests, where the possibility of structural changes is minimal 11. Although often under-recognized, patients are “experts” in the experience of their own illness. Thus, having patients involved from the start of the creation ensures that the desired features are implemented as intended and more rapidly 15,16.
Furthermore, PPI can also generate a sense of ownership, leading to increased motivation to use the mobile application (app) regularly. Therefore, it is importante to involve patients in all phases of the development and implementation of an app for asthma self-management.
OBJECTIVES
We aim to test a framework for the involvement of patients/carers in all phases of developing and implementing a mobile health app for asthma self-management.
METHODS AND ANALYSIS
This protocol was co-designed with six asthma patients, members of the ConectAR network 17. The ConectAR network is a community of people with chronic respiratory diseases, carers and citizens, researchers, and healthcare professionals interested in co-creating solutions with potential impact on healthcare to potentiate its quality through research and development processes.
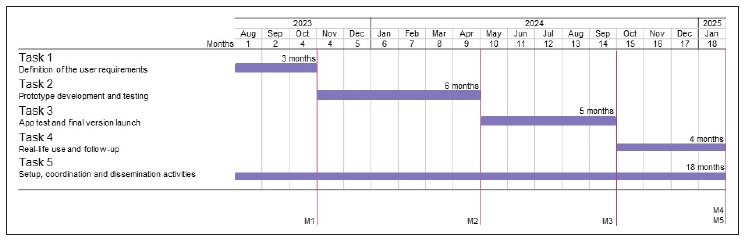
This protocol is based on a co-design technology development approach. This approach is iterative, involving multidisciplinary teams and incorporating user engagement as part of the design, testing, and implementation process. Telemedicine companies will also be invited to participate in the app development to foster its integration into clinical practice. Regular interactions between patients, developers and healthcare professionals will be a priority for user requirements definition (task 1) and iterative development and testing until the app’s final version (tasks 2 and 3). Patients/carers and healthcare professionals will be fully engaged in the real-life use study (task 4) and management and dissemination activities (task 5). The tasks and respective milestones schedule to be developed in the eighteen months of the project are presented in Figure 1.
Task 1. Definition of the user requirements
Task 1 aims to define the user requirements based on the experiences and needs perceived by patients with asthma and carers.
First, a brainstorming was held with six patients with asthma/carers to define the best method for user requirements definition. These patients contributed to this protocol and were included as authors. A web survey was found to be the most appropriate methodology by them and will be implemented. Apart from this core group, other members of the ConectAR network and the public will be invited to participate in the web survey by email, WhatsApp and social media. Participants will be asked to rate the importance of several features to be included in an app for asthma self-management. Some of the features to be included in the survey resulted from the brainstorm mentioned above and on the existing features of the apps already developed by the project team, such as therapeutic plan definition; symptom monitoring plan; lung auscultation; maximal expiratory flow sound collection during forced respiratory manoeuvre; cough sounds; medication intake reminder and register; report to show/send; pair support; timeline of events; gamification. More thorough research of the features available in apps for asthma and other chronic diseases that may fit the requirements for asthma self-management will also be included in the survey.
The survey will also collect demographic data and history of chronic respiratory disease. A draft of the survey is presented in Appendix 2. The results of this survey will be the ground for more meaningful, ongoing discussions to define the most important features to include in the app (anticipated to be among other those classified as “very important”/ “important” by at least 80% of participants).
At the end of task 1, we expect to have the list of the most important features to include in the first version of the asthma self-management app (milestone 1).
Task 2. Prototype development and testing
Task 2 aims to develop a prototype of a co-designed solution that supports asthma self-management using iterative methods and to test the prototype.
Based on the conclusions from task 1, we will develop a mobile app prototype. The development methodology will be centred around iterative development, where requirements and solutions evolve through collaboration between parties. For this, during the prototype phase, regular short meetings two weeks apart will be held among the patients/carers core group, developers, and healthcare professionals. It is expected that some features will be improved based on the existing features of the apps already developed by the project team rather than developed from scratch 18-20.
The Anonymous Digital Simulator will be used to create simulated app interfaces, detect interaction and usability problems along the process, and test users’ preferences and adherence factors to the app 21. This simulator will be used by the patients core group not only during the meetings, but also in their real-life context, to test if the app adapts to live situations.
Then, to test the prototype, at least 8 additional participants will be recruited (convenience sample) to allow the detection of 50-80% of app issues 22. The eligibility criteria to participate are 1) medical diagnosis of asthma or carer of a person with such diagnosis; 2) at least 18 years of age; 3) ability to use apps and access a mobile device with Internet. Participants will be invited to use the prototype and give structured feedback through web interviews and think-aloud methods. We anticipate using questionnaires such as the User Experience Questionnaire 23 and System Usability Scale 24.
The collected data will be analysed using a pseudo-anonymized database and used to refine the prototype.
At the end of task 2, we expect to have a beta version of the app available for further testing (milestone 2).
Task 3. App test and final version launch
Task 3 aims to test the app using testers-groups and refine the app’s beta version using the same iterative methodology. To test the app, at least 16 additional participants will be recruited through social media, patient organisations, and scientific societies using the same eligibility criteria as in task 2. Participants will be assigned into two groups with two researchers as observers, and instructions on how to use the mobile app will be provided. Participants will be invited to use the app and complete predefined tasks providing regular feedback about their experience with the mobile app.
The data collected from the participants regarding their experience with the mobile app will be used to refine it. During the refinement phase, regular short meetings two weeks apart will be held among the patients/carers core group, developers, and healthcare professionals. By the end of task 3, we expect to have the app’s final version (milestone 3).
Task 4. Real-life use and follow-up
Task 4 aims to disseminate the use of the app and measure its impact on asthma self-management. A website that explains the purpose of the study and provides information about the mobile app will be created.
We will invite participants from tasks 2 and 3 to use the app, as well as the public through social media, patient organisations and scientific societies. Then, all users will be invited to be included as participants in the study. For those who accept to participate, the app logs recording simple things like the number of times the app was used and task completion will be recorded. One month after the app download, participants will receive a short web survey on the app’s usability using User Experience Questionnaire and System
Usability Scale. The short-term effects of the mobile app will be explored through changes in disease monitoring (control measured by the measured by the Control of Allergic
Rhinitis and Asthma Test (CARAT; quality of life; exacerbations; utilisation of medical care; absenteeism; treatment adherence; etc.) and other outcomes relevant for patients/carers. By the end of this task we expect to have at least 30 users of the app answering the web survey (milestone 4).
Task 5. Setup, Coordination and Dissemination activities
Setup and coordination are critical for the management of the project, from its design to the results’ dissemination. To ensure the inclusion of patients’ perspective and experience, at least 3 patients/carers from the core group will actively participate in the study steering committee as team members. A kick-off meeting will establish general procedures and discuss the team members’ roles throughout the project. Videoconference meetings will be held monthly between the project team members.
During this task it is expected to have 1) the preparation and submission of applications to the ethics review board needed for all steps of this protocol; 2) the implementation of a collaborative shared online folder for the project team and other communication channels; 3) the definition of the requirements for the services that will be purchased, namely for the development of the app; 4) dissemination activities, including at least one original paper (milestone 5).
ETHICAL AND DIFFUSION ACTIVITIES
All study phases will be conducted according to the Declaration of Helsinki and Oviedo Convention. Participant’s personal data will be collected and treated according to the Portuguese law on Data Protection (Law number 67/98, October 26th) and the GDPR, executed and enforced in Portugal by Laws 58 and 59/2019. Specifically, to conduct these studies, ethical approvals will be obtained. Written informed consent will be obtained before any data collection from participants in accordance with GDPR requirements. Informed consent will include information to the participants regarding the types of personal and sensitive data that will be collected, how it will be processed, for what purposes and what security measures will be in place to provide for their privacy, as well as the conservation period or their elimination, after the project ends.
There are no risks anticipated for participants in relation to participation in this study. The participants’ privacy will be ensured by pseudo-anonymization techniques, which generate an anonymous univocal numeric code for each subject. The coding key will be encrypted, securely stored, and managed separately from participants’ personal and health-related data. All data analyses will be performed within the pseudo-anonymized versions of the original databases. The computers containing patient personal data will be subjected to password protection only by the Principal Investigator (PI) and the Co-PI. The storage of participants´ data will be managed through a centralised database structured to guarantee privacy and personal data protection. Only the PI will have access to the original database. This database will be saved on a server of the host institution, with daily backups and firewall protection.
The dissemination of the project results includes the writing and production of scientific outputs and diffusion to the public with the support of the ConectAR network and its social media channels. Scientific outputs will include at least one original paper and at least two scientific meetings about the results of the involvement of patients/ carers in all phases of developing and implementing a mobile health application for patients with asthma.
EXPECTED RESULTS AND POSSIBLE LIMITATIONS
By the end of the study, we expected to have the final version of the app for patients with asthma that meets patients’/carers’ needs and preferences and healthcare professionals’ concerns. The app will be designed based on patients’/carers’ needs and preferences and according to reliable recommendations for asthma self-management.
The co-design development, centred on user preferences and needs, offers the best user experience and interface design. The app will have the additional advantage of being available for patients/carers not only from Portugal but also from other Lusophone countries. This attractive app will support patients in self-management of their disease, keeping their asthma in control, having fewer asthma symptoms and enjoying life.
This study uses several usability methods, combining quantitative, qualitative and automated as recommended25. Despite the efforts to involve both patients/carers and healthcare professionals in the iterative development process of the app, its integration into routine clinical practice may not be possible due to the traditional clinical workflow and incompatibility with the informatic systems in the clinical setting. Nevertheless, telemedicine companies will be invited to collaborate in this project to foster future app integration in their systems.
This study protocol has some limitations that need to be acknowledged. This protocol is an effort to learn how to involve patients in digital health innovations meaningfully, yet the team has a short experience with PPI linked with digital solutions. Researchers and health professionals need to be aware of the challenges of PPI initiatives, namely the challenge of maintaining interest and preventing dropout rates of the patient co-researchers and dealing with time constraints (11). To ensure the feasibility of the study protocol in the proposed timeline, only the most relevant features will be included in the app. Thus, the app’s final version must be seen as an unfinished product that will continue to be improved in the following versions.
This study will be a framework for fully involving patients, carers and citizens in the iterative co-production development and implementation of a mobile health app for patients with asthma.