Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has spread globally rapidly since it was first identified in Wuhan, China, at the end of 2019.1,2 Initial reports during the early phase of the pandemic indicated that children and adolescents were relatively spared from severe disease manifestations. In April 2020, a cluster of eight previously healthy children presenting with fever, hyperinflammatory shock, ventricular dysfunction, and multiorgan involvement with a temporal association to SARS-CoV-2 infection was reported in the United Kingdom.3-5 Subsequently, similar cases were also reported in Europe, North America, Asia, and Latin America.6) Although this previously unreported condition and Kawasaki disease (KD) share similar presentations, they differ epidemiologically, with KD mostly affecting younger children compared with this syndrome, which has a reportedly older age of onset.1,8,9 In addition, both affect different races in different proportions.1 For these reasons, and given the recent epidemiological context of COVID-19 infection, suspicion was raised that they were two distinct entities.2
On May 14, 2020, the United States Centers for Disease Control and Prevention (CDC) named this syndrome Multisystem Inflammatory Syndrome in Children (MIS-C) associated with COVID-19 and introduced a case definition. Subsequently, the World Health Organization and the Royal College of Pediatrics and Child Health introduced their own case definition.4-6 All three definitions included the presence of fever, laboratory evidence of inflammation, and multisystem organ involvement without plausible diagnoses, as well as evidence of COVID-19 infection or recent exposure to the disease. The duration of fever, criteria for organ involvement, and need for documentation of SARS-CoV-2 infection differ between definitions.5
Variable presentations of MIS-C have been described, with features resembling macrophage activation syndrome, KD, Kawasaki shock syndrome, or toxic shock syndrome.1,2 These patients have laboratory evidence of cytokine storm, which can frequently lead to multiorgan failure requiring intensive care.2,6 MIS-C’s rare form of presentation seems to develop after COVID-19 infection rather than during its acute stage.6 Because the time from infection to onset of MIS-C symptoms varies, a small number of patients with MIS-C present with positive reverse transcription polymerase chain reaction (RT-PCR) testing for SARS-CoV-2. Most have known family exposure or serological evidence of previous infection (presence of immunoglobulin [Ig] G antibodies). These results suggest that a post-infectious immune response may be responsible for MIS-C.5
The knowledge of this syndrome’s pathophysiology is evolving, with the clinical spectrum and optimal treatment regimen not fully understood yet.4,6) No widely established management guidelines are available. In several centers, the treatment protocol is based on specific symptoms and previous treatment of similar conditions, such as KD or COVID-19, with guidelines for adult patients used as reference in the latter.6 A multidisciplinary team approach should be used in the management of patients with MIS-C.5,6
Case report
A fully immunized 10-year-old Caucasian girl with a history of pulmonary valve stenosis with percutaneous intervention at the age of four months, without hemodynamic repercussion or other comorbidities (including obesity), was admitted to the Pediatric Emergency Department with fever, headache that did not cease in apyrexia or with analgesia, vomiting and refusal to eat with two days of evolution. On admission, she was pale but hemodynamically stable and apyretic. On cardiac auscultation, previously recognized systolic heart murmur (grade II/VI) was identified. The physical examination additionally showed mild pharyngeal erythema. No other pathological findings were observed, and the neurologic exam was normal. Rapid group A streptococcus antigen and RT-PCR for SARS-CoV-2 were performed, both with negative result. The laboratory assessment (Table 1) showed lymphopenia (500/mm3), mild thrombocytopenia (116,000/mm3), and positive C-reactive protein (CRP; 70.3 mg/L). The child was discharged with a medical appointment scheduled for clinical and analytical follow-up.
On the third day of disease, the girl again presented with the previous symptoms and non-pruriginous rash of sudden onset over the auricular and retroauricular areas with caudal progression. She also complained of worsening headaches, myalgia, and fatigue and was lethargic. Recent infections, hemorrhagic, respiratory, or urologic symptoms, or use of any chronic medication were denied. Recent traveling or contact with patients with COVID-19 or similar symptoms were also denied. On admission, the girl was feverish and tachycardic but with normal blood pressure. On physical examination, she presented with non-petechial, macular erythematous rash dispersed over both ears, trunk, legs, and pubic area but not over feet or palms, and mild pharyngeal erythema. Meningeal signs were negative. No other pathological findings were evident. During observation, the girl had one episode of non-bloody, non-mucoid diarrhea. Laboratory assessment was repeated (Table 1), showing lymphopenia (500/mm3), decreased thrombocytopenia (63,000/mm3), and increased CRP (124 mg/L). RT-PCR for SARS-CoV-2 was also repeated, this time with a positive result. The girl was admitted to the Pediatric Ward for clinical observation and started on intravenous ceftriaxone (80 mg/kg/day) for suspected septic shock and fluid replacement.
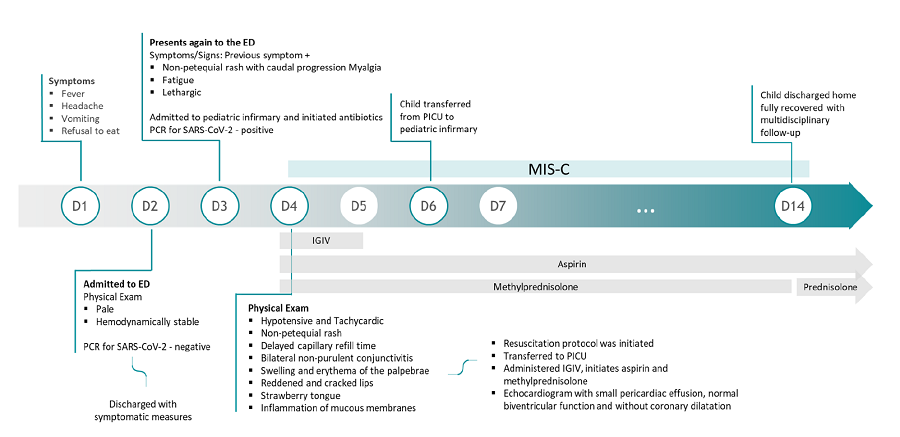
On the fourth day, the patient maintained initial symptoms and mentioned a subjective perception of decreased urinary output. She was hypotensive (74/34 mmHg; systolic and diastolic blood pressure inferior to the reference 5th percentile for age, sex, and height) and tachycardic (133 bpm). The physical exam showed the previously described rash (Figures 1.A and 1.B), delayed capillary refill time (three seconds), mild bilateral non-purulent conjunctivitis (Figure 1.C), swelling and erythema of the palpebrae and nose (Figure 1.D), reddened and cracked lips, strawberry tongue, and inflammation of mucous membranes (Figures 1.D and 1.E). Laboratory assessments (Table 1) showed mild normochromic normocytic anemia (hemoglobin 11.1 g/dL; mean corpuscular volume 80.6 fL; mean corpuscular hemoglobin 28.5 pg), increasing lymphopenia (300/mm3), and thrombocytopenia (58,000/mm3). CRP (124.0 mg/L), d-dimer (7,500 ng/mL), lactate dehydrogenase (345 UI/L), erythrocyte sedimentation rate (28 mm), procalcitonin (113.48 mg/L), and ferritin (596.60 ng/mL) were elevated. Liver enzymes, aspartate transaminase (ALT; 70 UI/L), and alkaline phosphatase (ALP; 62 UI/L) were mildly elevated, with hypoalbuminemia (2.6 g/dL) and decreased total proteins (5.4 g/dL). Coagulation tests were normal. The laboratory study additionally revealed increased troponin (0.015 ng/mL) and pro-brain natriuretic peptide 4528 pg/mL) and hypertriglyceridemia (219 mg/dL). Urine analysis was normal, and blood and urine cultures were negative. Chest x-ray showed no alterations.
Given the clinical and analytical evolution and positive SARS-CoV-2 testing, the diagnosis of MIS-C was considered. SARS-CoV-2 serologies were performed, with results showing strongly positive IgG and borderline positive IgM.
Due to hemodynamic instability, fluid resuscitation with normal saline fluid was provided, without hemodynamic improvement. Intravenous dexamethasone was administered, with normalization of blood pressure, capillary refill time, and heart rate, and improvement of rash, eyelid erythema, inflammation of mucous membranes, and other inflammatory signs (Figures 2.A, B and C). The patient was transferred to the Pediatric Intensive Care Unit , where she received one dose of intravenous immunoglobulin (IVIG - 2 g/kg), intravenous methylprednisolone (1 mg/kg/day), and aspirin at anti-inflammatory dose (30 mg/kg/day). She was also assessed in Pediatric Cardiology, with clinical examination and echocardiogram showing a status of post-pulmonary artery dilatation, with free pulmonary insufficiency without significant gradient in the right ventricular outflow tract, small pericardiac effusion, normal biventricular function, and absence of coronary dilatation or aneurism. On the fourteenth day of disease, the child was discharged home, fully recovered, with a corticoid tapering scheme and aspirin in anti-inflammatory dose (Figure 3). Pediatric Cardiology and Infectious and Immunodeficiency Diseases appointments were scheduled for surveillance and follow-up.
Table 1 Analytical evolution of the presented case
| D2 | D3 | D4 | D14 | |
| Hemoglobin (g/dL) | 12.7 | 12.1 | 11.1 | 11.6 |
| Leucocytes (/uL) | 4400 | 6500 | 4600 | 13970 |
| Neutrophiles (/uL) | 3500 | 5800 | 4100 | 11300 |
| Lymphocytes (/uL) | 500 | 500 | 300 | 1900 |
| Platelets (/uL) | 116000 | 63000 | 58000 | 528000 |
| Urea (mg/dL) | 22 | 28 | 28 | 38 |
| Creatinine (mg/dL) | 0.5 | 0.51 | 0.62 | 0.36 |
| AST (U/L) | 81 | 61 | 70 | 35 |
| ALT (U/L) | 101 | 57 | 62 | 71 |
| CRP (mg/L) | 70.3 | 124 | 124.0 | 2.36 |
| ESR (mm/hr) | - | 28 | - | |
| Ferritin (ng/mL) | - | 863 | 391 | |
| Lactate dehydrogenase (UI/L) | - | - | 596 | 187 |
| Albumin (g/dL) | - | - | 2.6 | 3.57 |
| Troponin (ng/mL) | - | - | 0.052 | 0.003 |
| ProBNP (pg/mL) | - | - | 4528.0 | 269 |
| D-dimer (ng/mL) | - | - | 7500 | 463 |
ALT- alanine transaminase; AST- aspartate transaminase; CRP - C-reactive Protein; D - day …; ESR - Erytrochyte sedimentation rate; Pro-BNP - Pro-brain natriuretic peptide
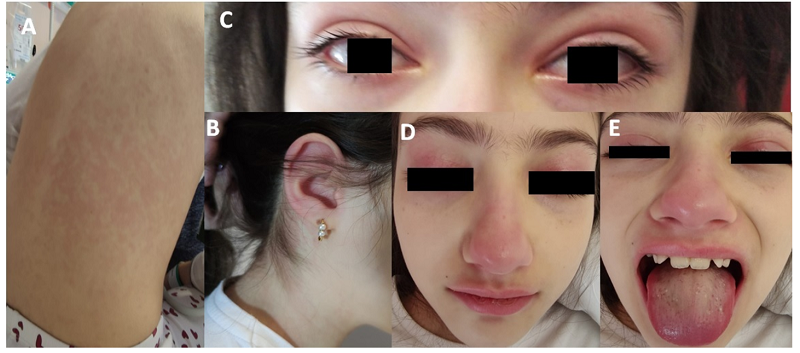
Figure 1 Clinical findings on physical examination. A and B - Non-petechial, macular erythematous rash; C - Mild bilateral non-purulent conjunctivitis; D - Swelling and erythema of the palpebrae and nose; E - reddened and cracked lips, strawberry tongue, and inflammation of mucous membranes
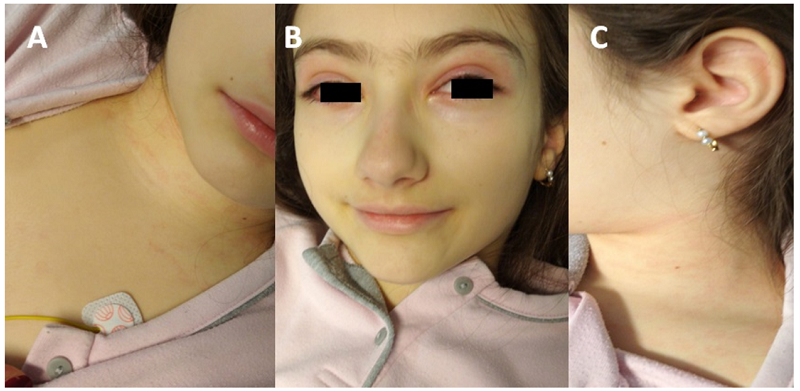
Figure 2 Improvement of physical findings after resuscitation therapy. A, B, and C - Improvement of rash, eyelid and nose swelling and erythema, inflammation of mucous membranes, and other inflammatory signs
Discussion
Although not fully established yet, epidemiologic data suggest that SARS-CoV-2 is the likely etiological agent of the newly recognized MIS-C.
The pathophysiology of MIS-C is still largely unknown, but it is believed to be a post-infectious immune-mediated phenomenon. A growing body of evidence suggests that MIS-C is not mediated by viral invasion but concurs with the development of acquired immune response to SARS-CoV-2 that typically occurs three to four weeks after the acute infection.6,10 The delay in the presentation of the condition regarding the pandemic curve, low proportion of SARS-CoV-2-positive cases by RT-PCR, and high proportion of antibody-positive cases support the hypothesis that MIS-C is not mediated by direct viral invasion but instead concurs with the development of acquired immune response to SARS-CoV-2.4,6
In the present case, the clinical presentation met criteria for MIS-C with incomplete KD phenotype. Epidemiologically, the case is in agreement with what has been reported for this new syndrome. Despite the patient denying previous symptoms, previous SARS-CoV-2 infection cannot be excluded since it is reported that children can develop MIS-C despite an asymptomatic course of COVID-19.9 Although the most common findings in MIS-C are negative RT-PCR and positive antibody serologies for COVID-19, the presence of positive RT-PCR, as in the present case, is not synonymous of a current infection. It is acknowledged that COVID-19 patients can have detectable SARS-CoV-2 RNA in upper respiratory tract specimens for weeks after the onset of symptoms.11 Recurrently positive RT-PCR for SARS-CoV-2 after at least one negative test has also been reported.12 Prolonged RNA detection does not necessarily mean prolonged infectiousness. Isolation of infectious virus from upper respiratory specimens more than ten days after symptom onset has only rarely been documented in patients with non-severe disease presentation.12 The IgG-positive serologies found in the present case also favor a previous COVID-19 infection. These findings support the need to include SARS-CoV-2 serologies in the analytical study of patients with symptoms compatible with MIS-C.1,13
MIS-C clinical presentation is very distinct from COVID-19 acute presentation. While respiratory symptoms are common in the acute form of COVID-19, respiratory symptoms are rare in MIS-C, with fever, gastrointestinal symptoms, and rash prevailing.4,8 Cardiovascular symptoms are also common in MIS-C, with ventricular dysfunction frequently reported and coronary artery dilation and aneurysm, arrhythmia, and elevated NT-pro-BNP and troponin also documented. Although this patient showed an important analytical cardiac dysfunction (elevated troponin and pro-BNP), Pediatric Cardiology and echocardiogram assessment showed no alterations. Still, cardiac follow-up is crucial in these cases since some series suggest that progression of coronary artery aneurysm may occur following discharge.4-6 Common analytical findings include a significant elevation of inflammatory markers, such as CRP, erythrocyte sedimentation rate, procalcitonin, and ferritin. Hyponatremia, acute kidney injury, and hypoalbuminemia can also be identified, suggesting generalized inflammation, and neutrophilia, lymphopenia, thrombocytopenia, elevated D-dimers, and low fibrinogen levels have also been reported.1,9 Except for acute kidney injury, all these findings were identified in the present case.
MIS-C may share features with septic and toxic shock syndrome, as observed in the present case.1,4 Due to this, empiric antibiotics were started, following CDC guideline recommendations,13and discontinued when blood and urine culture results were negative.
Due to the patient’s clinical decline, fluid resuscitation was warranted. However, resistant hypotension ─ a common feature in MIS-C ─ developed.6 Significant clinical improvement was achieved with the administration of dexamethasone, with no need for vasopressor therapy. The role of glucocorticoids in MIS-C remains uncertain, but a recent retrospective study showed that children receiving glucocorticoids in association with IVIG have more favorable outcomes compared to children receiving only IVIG as initial therapy.1,6,14 In KD, glucocorticoid administration has been shown to decrease the risk of coronary abnormalities.6 Methylprednisolone has been the chosen glucocorticoid in most published reports and guidelines, but dexamethasone has also proven effective and is thus suggested as an option in the treatment of MIS-C. Studies suggest that this agent can help suppress the immune response and subsequent inflammatory disorders in patients with MIS-C. (6,14,15
Due to the similarities between MIS-C and KD, the same treatment protocol is often used for both conditions. Most children have been treated with IVIG, and several have also received adjunctive high-dose steroids, mostly with favorable outcomes.1,6,13 In this clinical case, the presentation was compatible with incomplete KD phenotype, and treatment response was also favorable, with the patient showing good clinical and analytical outcomes.
Remdesivir, a drug shown to reduce the duration of COVID-19 illness by decreasing viral RNA production, was not included in the treatment protocol of this case because the child was not considered to be in the acute phase of the disease. This is also the reason why this drug is not widely recommended in MIS-C.6
Despite being a rare manifestation of SARS-CoV-2 infection, the evolution of MIS-C is fast and often leads to the need for intensive care or, in rare cases, death. Given the current epidemiological setting, clinicians should be particularly aware of the signs and symptoms of this syndrome and promptly institute treatment once suspicion is raised. The initial approach to these patients should be multidisciplinary, involving the Pediatric Infectious Disease, Cardiology, Immunology, and Intensive Care units. As long-term complications of MIS-C are still unknown, close follow-up, particularly through regular cardiac assessment, is recommended.
List of abbreviations
SARS-CoV-2 - Severe acute respiratory syndrome coronavirus 2
KD - Kawasaki Disease
CDC - United States Center of Disease Control
MIS-C - Multisystem Inflammatory Syndrome in Children
RT-PCR - Real-time Polymerase Chain Reaction
CRP - C-reactive Protein
IVIG - Intravenous Immunoglobulin