Introduction
Type 1 diabetes mellitus (T1DM) is one of the most prevalent chronic diseases of childhood.1 Its pathogenesis is not completely understood. It is an organ-specific autoimmune disease that results from the interaction between genetic polymorphisms and environmental triggers. Autoreactive T-cells are activated and destroy insulin-producing pancreatic β-cells, leading to insulin deficiency, glucose metabolism dysregulation, and lifelong insulin dependence.1,2
At the time of diagnosis, patients show variable residual β-cell function and endogenous insulin secretion. A large body of evidence indicates that some individuals show persistent β-cell residual function even decades after diagnosis.3 Greater residual β-cell function is associated with enhanced glycemic control over time and hence with a reduction in the risk of acute and chronic complications.4 Therefore, therapeutic interventions aiming to preserve or restore β-cells may be clinically relevant. None of the therapeutic approaches investigated to date has shown durable effects, but the field has gained valuable insights.2 Patients with better residual β-cell function are more likely to respond to therapies that prevent pancreatic damage.
Pancreatic β-cell function can be measured by serum C-peptide levels. After cleavage of proinsulin, insulin and C-peptide are released in equal amounts. C-peptide testing is more reliable than insulin testing due to the greater stability of C-peptide levels: C-peptide degradation rate is slower (half-life of 20-30 minutes, compared to 3-5 minutes for insulin), and it is cleared from circulation at a constant rate, whereas insulin has a variable clearing rate. A simple method to evaluate β-cell function is dosing fasting venous C-peptide levels.5
Factors potentially affecting C-peptide levels at T1DM diagnosis are not established. Therefore, the present study aimed to identify clinical and laboratory factors associated with fasting C-peptide levels at T1DM diagnosis.
Material and methods
This was a retrospective study of children and adolescents with T1DM admitted to a reference center in the north of Portugal over nine years (August 2010−July, 2019). Ethical approval was obtained from the hospital’s Ethics Committee. Study inclusion criteria comprised subjects with newly diagnosed T1DM during the study period, aged between six months and 17 years and 364 days, and with fasting C-peptide levels measured at diagnosis. Subjects were selected from an institutional database of pediatric diabetic patients.
Study data were retrospectively collected from patients’ medical records and included age, gender, pubertal stage, season, previous symptom duration, fasting C-peptide levels, body mass index (BMI) z-score, hemoglobin A1C (HbA1C), pH, bicarbonate levels, presence of diabetic ketoacidosis (DKA), positivity for diabetes autoantibodies (glutamic acid decarboxylase 65 autoantibodies [GADA], tyrosine phosphatase-like insulinoma antigen 2 [IA2], insulin autoantibodies [IAA], and β-cell specific zinc transporter 8 autoantibodies [ZnT8]), and family history of T1DM at diagnosis.
Fasting C-peptide levels were measured on the days immediately after diagnosis, as soon as metabolic control was restored. The cut-off of 0.6 ng/mL (0.2 nmol/L) was assumed to define preserved C-peptide levels, indicating residual β-cell function.5
BMI was calculated using the weight at the first medical appointment after recovery from acute T1DM presentation (DKA or hyperglycemia). BMI z-score was determined using World Health Organization (WHO) AnthroPlus software. Overweight and obesity were diagnosed according to WHO criteria.
HbA1C was determined by ion-exchange high-performance liquid chromatography (HPLC), a National Glycohemoglobin Standardization Program (NGSP)-certified method.
DKA was defined by the presence of hyperglycemia (blood glucose > 200 mg/dL [11 mmol/L]), venous pH <7.3 or serum bicarbonate <15 mmol/L AND ketonemia (blood β-hydroxybutyrate ≥3 mmol/L), or moderate or large ketonuria.6
Statistical analysis
All statistical analyses were conducted using IBM SPSS® Statistics v. 19.0. The Kolmogorov-Smirnov test (with Lilliefors significance correction) was applied to investigate data’s normal distribution. Values were reported as mean and standard deviation (SD) for variables with normal distribution or median and interquartile range (IQR) for those without normal distribution.
Spearman’s rank correlation was used to test a possible association between C-peptide levels and continuous variables, including age, previous symptom duration, BMI z-score, pH, bicarbonate levels, and HbA1C. Mann-Whitney U test or Kruskal-Wallis test were used to compare C-peptide levels according to nominal variables classification, namely gender, presence of puberty, seasonality, presence of overweight/obesity, diabetes autoantibodies positivity, and family history of T1DM. A p-value <0.05 was considered statistically significant.
Results
Among 160 patients diagnosed with T1DM during the study period, 109 (64 boys and 45 girls) were enrolled in the study. The remaining 51 patients were excluded for not having fasting C-peptide levels measured at diagnosis. The mean decimal age at diagnosis was 8.6 years (minimum [min] 0.76 years, maximum [max] 17.33 years, SD ± 4.66), and 29.4% of patients had already reached puberty. The median fasting C-peptide level was 0.46 ng/mL (min <0.01 ng/mL, max 1.99 ng/mL, IQR 0.45 ng/mL). A fasting C-peptide level ≥ 0.6 ng/mL was recorded in 33.9% of study participants, indicating residual β-cell function. The mean BMI z-score was 0.09 (min 3.89, max 4.48, SD ± 1.51), and overweight or obesity was identified in 12% of cases. At T1DM presentation, patients reported a median duration of previous symptoms of 15 days (min 0 days, max 180 days, IQR 23 days), and 22% presented with DKA. The median HbA1C was 10.85% (min 7.2%, max 17%, IQR 2.9%). Regarding seasonality, 62.4% of cases were diagnosed in the autumn/winter months (September-February), while 37.6% were diagnosed in the spring/summer months (March-August). Positive family history of T1DM was identified in 26.2% of subjects (12.2% in first-degree relatives and 14% in second-degree relatives). Regarding diabetes autoantibodies, 102 of 109 patients were tested for GADA, 58% of whom were positive; 43 of 109 patients were tested for IA2, 65% of whom were positive; 79 of 109 patients were tested for IAA, 32% of whom were positive; and 44 of 109 patients were tested for ZnT8, 57% of whom were positive.
Regarding gender, no statistically significant differences were found in fasting C-peptide levels between boys and girls (median 0.46 ng/mL, IQR 0.4 vs. median 0.5 ng/mL, IQR 0.52; p=0.46).
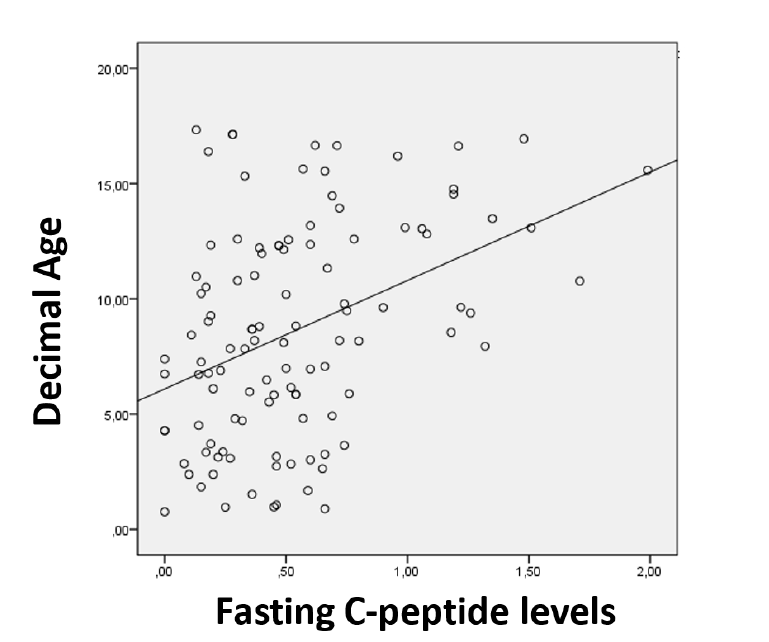
A positive correlation was found between fasting C-peptide levels and age at diagnosis (p <0.001), suggesting higher residual β-cell function in older subjects (Figure 1). Pubertal individuals showed significantly higher fasting C-peptide levels compared to prepubertal counterparts (median 0.68 ng/mL, IQR 0.67 vs. median 0.4 ng/mL, IQR 0.39; p <0.001).
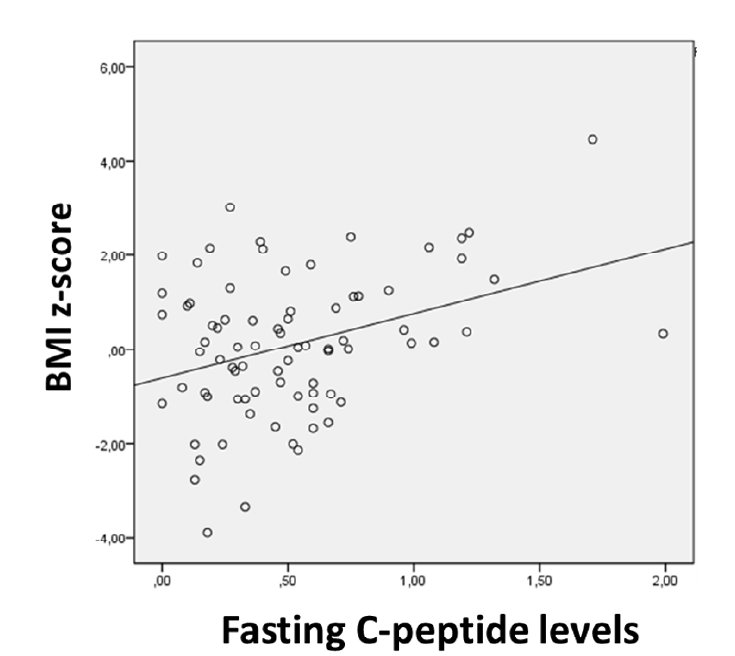
When investigating a possible association between fasting C-peptide levels and BMI z-score, a positive correlation was found (p=0.041), suggesting an improved residual β-cell function in children/adolescents with higher BMI z-score (Figure 2). Significantly higher fasting C-peptide levels were measured in overweight/obese subjects compared to average-weight counterparts (median 0.76 ng/mL, IQR 0.73 vs. median 0.41 ng/mL, IQR 0.41; p=0.008). No statistically significant difference was found in the age of overweight/obese subjects and the remaining study participants (mean 10.7 years, SD ± 2.36 vs. mean 8.51 years, SD ± 4.71; p=0.084). No correlation was found between age and BMI z-score (p=0. 725).
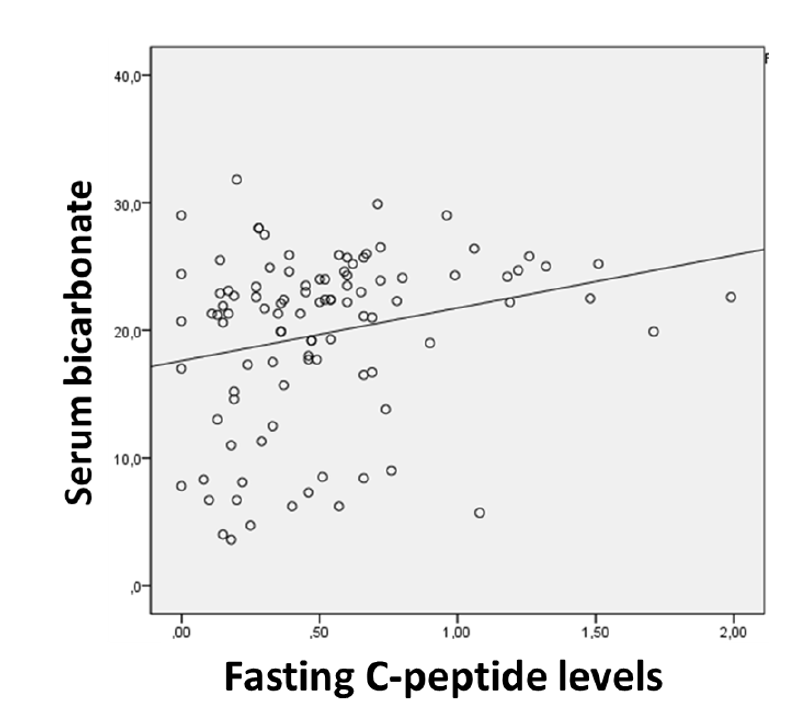
A positive correlation was identified between fasting C-peptide levels and serum bicarbonate levels at T1DM presentation (p=0.005), indicating that higher C-peptide levels are related to higher bicarbonate levels (Figure 3). Bicarbonate levels did not correlate with age at diagnosis (p=0.367) or BMI z-score (p=0.622). No correlation was found between fasting C-peptide levels and pH (p=0.649), HbA1C (p=0.988), or previous T1DM symptom duration (p=0.454).
No differences were found in fasting C-peptide levels according to diabetes autoantibodies status (positive vs. negative). The median (IQR) fasting C-peptide levels identified for positive versus negative autoantibodies were 0.49 ng/mL (IQR 0.51) versus 0.46 ng/mL (IQR 0.42), p=0.91 for GADA; 0.5 ng/mL (IQR 0.44) versus 0.4 ng/mL (IQR 0.38), p=0.17, for IA2; 0.46 ng/mL (IQR 0.37) versus 0.5 ng/mL (IQR 0.64), p=0.234 for IAA; and 0.43 ng/mL (IQR 0.39) versus 0.46 ng/mL (IQR 0.46), p=0.924 for ZnT8, respectively.
Similar fasting C-peptide levels were detected in patients with and without T1DM family history (median 0.4 ng/mL, IQR 0.49 vs. median 0.56 ng/mL, IQR 0.41; p=0.251). Similarly, no differences were found in residual β-cell function according to the family degree of the relative with T1DM (p=0.089).
Similar fasting C-peptide levels were identified in children/adolescents diagnosed with T1DM in autumn/winter compared to spring/summer months (median 0.5 ng/mL, IQR 0.38 vs. median 0.42 ng/mL, IQR 0.55; p=0.423).
Discussion
The present study documented a wide variety of C-peptide levels at T1DM diagnosis, in agreement with what has been reported in the literature,3 showing that patients were diagnosed in different stages in the course of the disease. Some had considerable residual β-cell function. Fasting C-peptide levels within the normal range (≥ 0.6 ng/mL) were documented in 33.9% of subjects, suggesting that other potential factors may be involved in disease manifestation.
In this study, fasting C-peptide levels positively correlated with age, BMI z-score, and serum bicarbonate levels. Pubertal and overweight/obese individuals showed significatively higher fasting C-peptide levels.
The positive correlation observed between fasting C-peptide levels and age has been reported in multiple studies.4,7,8 A better residual β-cell function in older children/adolescents can possibly be explained by age-related differences in T1DM pathology. Younger children exhibit decreased immune regulatory function, increased engagement and trafficking of autoreactive CD8+ T cells, and greater β-cell vulnerability. In addition, pancreatic islet damage is probably more pronounced in younger children9 and β-cell mass is lower since adult β-cell mass is not reached until early adolescence.10 Another possible explanation is a later diagnosis in younger children due to difficulties in caregivers recognizing T1DM symptoms.
The higher C-peptide levels in pubertal compared to prepubertal children observed in this study is consistent with findings in the literature.8 Achieving total β-cell mass in early adolescence can also contribute to this observation. Puberty endocrine metabolic changes lead to the development of hyperglycemia and diabetes at an earlier stage of pancreatic damage. Pubertal growth spurt contributes to insulin resistance as a result of increased growth hormone and increases insulin demands, accelerating the emergence of dysglycemia.11
Findings of a positive correlation between fasting C-peptide levels and BMI z-score and higher C-peptide levels in overweight/obese individuals have also been documented by other authors.7,8 In this study, BMI z-score did not correlate with age at diagnosis, and no age differences were found between overweight/obese subjects and other study participants. This observation suggests a direct impact of BMI z-score in residual β-cell function. The likely explanation for this relation is insulin resistance. Since hyperglycemia arises when insulin requirements surpass its production by the body, insulin-resistant children/adolescents will present with hyperglycemia with more residual insulin production than insulin-sensitive counterparts. It is possible that insulin resistance accelerates T1DM presentation.8
DKA is a condition characterized by severe insulin deficiency. Extremely low C-peptide levels are expected, as confirmed in some studies.4 The positive correlation found in this study between fasting C-peptide levels and bicarbonate levels has not been previously reported but is probably related to previous observations of lower C-peptide levels in children with DKA compared to children without the condition. However, no correlation was found between fasting C-peptide levels and pH, which can be possibly explained by the lower stability of pH values, as it is dependent on multiple compensatory mechanisms. Unlike what has been reported in several other studies, in the present study HbA1C did not correlate with C-peptide levels. This was an unexpected finding since residual β-cell function is associated with better metabolic control, and thus a negative correlation was anticipated. Previous T1DM symptom duration was also not indicative of the extension of β-cell loss in this study, but this was not surprising given that symptom duration is dependent on patients’/caregivers’ perception.
No gender differences were found in this study, and the reports in the literature of different residual β-cell function according to sex are inconsistent. Heterogeneity in age or BMI z-score could explain different C-peptide levels in boys and girls in some studies, but this was not always verified. Some authors proposed the existence of sex-related differences in the disease process at diabetes diagnosis,7 but the results obtained here do not support this theory.
Diabetes autoantibody positivity reflects the autoimmune attack suffered by the pancreas. A more pronounced β-cell loss would be expected in patients with positive T1DM autoantibodies, as a large Chinese study demonstrated.12 In addition, the presence of several autoantibodies should be associated with lower C-peptide levels. However, no significant differences were found in C-peptide levels between patients with positive and negative autoantibodies in the present study. A comparison of patients with different numbers of positive antibodies or different antibody titers was not possible because laboratorial techniques used to measure antibodies suffered alterations over the study period and not all patients had all antibodies measured.
Family history can partly explain the influence of genetic polymorphisms in residual β-cell function. No differences were found in C-peptide levels in subjects with a positive and negative family history or in subjects with different degree relatives with T1DM. These findings are consistent with the absence of genetic influence on β-cell function at T1DM diagnosis. This correlation was not reported in other studies.
No statistically significant differences were found in C-peptide levels according to the season of diagnosis, making a possible role of environmental determinants in residual pancreatic islet function unlikely.
Beyond the relevance of residual β-cell function at T1DM diagnosis in glycemic control, its loss rate is crucial. Some of the clinical factors found to correlate with C-peptide levels in the present study had already been associated with a slower decrease rate of C-peptide levels, such as older age and higher BMI score. This is an evolving field.4,10
This study has some limitations that should be acknowledged. The only parameter used to assess adiposity was BMI. The weight used to calculate BMI was measured after diagnosis, as there were no data regarding body weight in the prediabetic period. Additionally, not all patients had their autoantibody levels measured at diagnosis.
Conclusions
In this study, older, pubertal, and overweight/obese subjects presented higher C-peptide levels. Their improved residual β-cell function makes them better candidates for therapies that preserve islet pancreatic cells, currently under investigation. In the acute setting, the best biochemical parameter correlated to residual β-cell function is probably serum bicarbonate. T1DM diagnosis is more challenging in younger patients, and their residual β-cell function is lower. An improved screening and diagnostic approach is relevant in this group to improve the chances for therapeutic intervention. This study provides support on how clinical and laboratory factors affect residual insulin production in T1DM, but further research is needed to change the disease course and optimize treatment outcomes.
Abbreviations and acronyms
BMI |
-Body mass index |
DKA |
-Diabetic ketoacidosis |
GADA |
-Glutamic acid decarboxylase 65 autoantibodies |
HbA1C |
-Hemoglobin A1C |
IAA |
-Insulin autoantibody |
IA2 |
-Tyrosine phosphatase-like insulinoma antigen 2 |
IQR |
-Interquartile range |
Max |
-Maximum |
Min |
-Minimum |
SD |
-Standard Deviation |
T1DM |
-Type 1 diabetes mellitus |
ZnT8 |
- β-cell-specific zinc transporter 8 autoantibodies |
Authorship
Vera Gonçalves - Conceptualization; Data curation; Formal Analysis; Investigation; Methodology; Visualization; Writing - original draft; Writing - review & editing
Liliana Teixeira - Conceptualization, Investigation, Methodology, Visualization, Writing - original draft, Writing - review & editing
Joana Freitas - Conceptualization; Methodology; Writing - review & editing
Maria João Oliveira - Conceptualization; Methodology; Writing - review & editing
Teresa Borges - Conceptualization; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing - original draft, Writing - review & editing