Introduction
Osteoarticular pain is a common complaint in children. Although the etiology is mostly benign, a thorough diagnostic evaluation is crucial, as the condition can be caused by a wide range of diseases with different manifestations and severity and variable therapeutic response. Osteomyelitis is a bone infection most commonly caused by bacteria that usually affects long tubular bones. The exact incidence of acute osteomyelitis in children is unknown. The estimated incidence is 8: 100,000 children/year. The condition has a male predominance (2:1) and 50% of cases occur in children under the age of five years.1 Pelvic osteomyelitis is rare in childhood, accounting for 3−8% of cases. The most common location is the ilium (38%), with the pubis (14%) and acetabulum (12%) less commonly affected.1 Infectious (osteomyelitis pubis) and non-infectious (osteitis pubis) inflammation of the symphysis pubis are distinct entities with similar presentation. Bacteria can enter the bone marrow through the bloodstream or spread from nearby tissues. Osteomyelitis can be classified as acute, subacute, or chronic based on the time from onset of symptoms to diagnosis. In children, osteomyelitis is primarily hematogenous in origin and acute in nature.1 The authors describe the case of a child with a rare cause of pubic pain, antalgic gait, and fever, highlighting that early recognition of the condition may have prognostic implications.
Case report
A previously healthy nine-year-old girl was admitted to the Pediatric Emergency Department due to a two-week history of bilateral pain in the medial side of the thigh associated with a one-week history of fever and pubic pain. Muscle pain began as persistent pain in the right adductor region with progressive involvement of the contralateral side and pubic region, followed by impaired gait.
One week before the onset of symptoms, the girl suffered a fall with subsequent trauma to the left thigh. Physical examination on admission revealed tenderness overlying the symphysis pubis without abnormalities of the knees or ankles. There were no palpable masses in the pelvis or lower extremities. Both passive and active hip movements were painful and limited, associated with an antalgic gait.
Initial laboratory workup revealed hemoglobin of 11.3 g/dL with a mean globular volume of 75 fL, total leukocyte count of 15.6 x 103 /uL, erythrocyte sedimentation rate of 36 mm/h (0-10 mm/h), and C-reactive protein (CRP) of 134.3 mg/L (range 0−3.5 mg/L), consistent with an inflammatory syndrome. Serum creatine kinase and lactate dehydrogenase were within normal range for age. Blood and urine cultures were negative. In addition, there were no pathologic findings on the anteroposterior pelvic radiograph. The girl was admitted to the hospital. Magnetic resonance imaging (MRI) showed evidence of bone marrow edema with increased T2 signal and decreased T1 signal in both pubic bodies, with a small amount of pubic symphysis effusion associated with lower edema of the adductors and rectus abdominis, consistent with osteitis pubis. These findings were more pronounced on the right side with disruption of the cortical bone in the anteroinferior part of the pubic body, reflecting a focus on osteomyelitis pubis. MRI also showed a 17 x 9 mm intramuscular collection in the upper and anterior slopes of the right adductor region, consistent with an abscess (Figures 1A-B).
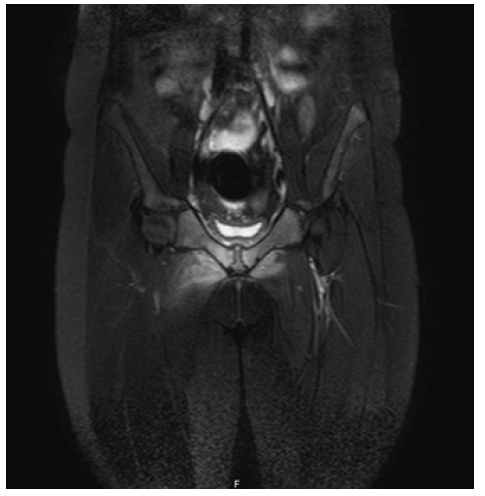
Figure 1A Coronal T2-weighted MRI of the pelvis showing bone marrow edema and disruption of the cortical bone in the anteroinferior part of the pubic body, reflecting a focus on osteomyelitis pubis
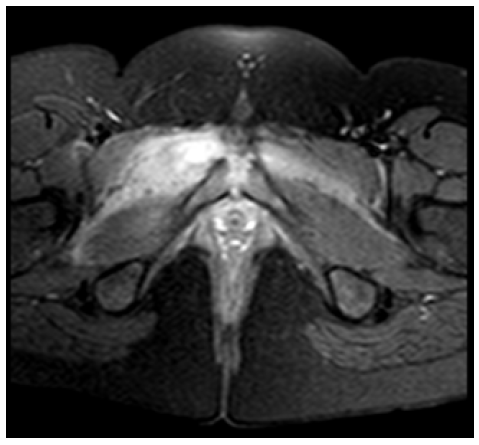
Figure 1B Axial T1-weighted pelvic MRI showing interfascial and pelvic muscle edema associated with a 17 x 9 mm intramuscular collection in the upper and anterior slopes of the right adductor region, consistent with an abscess
The diagnosis of osteomyelitis pubis was established, and intravenous (IV) treatment with flucloxacillin and ceftriaxone was started, with initial clinical improvement. The girl was apyretic after the 3rd day of IV antibiotic therapy. However, she relapsed with fever on day 12, and analytical and imaging evaluation was performed to exclude complications. Laboratory workup revealed hemoglobin of 10.2 g/dL with a mean globular volume of 73.5 fL, total leukocyte count of 4.5 x 103 /uL, erythrocyte sedimentation rate of 67 mm/h (0-10 mm/h), and CRP of 33.3 mg/L (range 0−3.5 mg/L). Blood cultures were negative. An ultrasound was performed, which showed persistence of slightly increased globosity of the right adductor muscles without nodules in its thickness. Compared to the previous MRI, the medullary edema persisted and the joint effusion regressed. In addition, de novo edema of the proximal portions of the adductor muscles, more extensive on the right side, was identified, which could translate into possible myositis and tendinopathy with continuity. Due to the clinical, analytical, and imaging deterioration and the association with the right adductor abscess, clindamycin was added with subsequent clinical improvement. Clinical and analytical improvement was seen after 21 days of hospitalization (21 days of flucloxacillin and nine days of clindamycin) when the patient was discharged. Since no etiologic agent was isolated and given the diagnosis of complicated pubic osteomyelitis with initial lack of clinical response, the patient was discharged with dual oral therapy (flucloxacillin and cefixime) after completing six weeks of antibiotic therapy. Six months after discharge, the girl remained asymptomatic.
Discussion
Recent studies have reported an increase in the incidence of osteomyelitis and, more worryingly, an increase in the severity of musculoskeletal infections in children. Studies indicate that the incidence of osteomyelitis increased by 11.7%, from 8.2 cases per 100,000 children in 2009 to 9.2 cases per 100,000 children in 2019.2 Factors contributing to the increase in musculoskeletal infections include the emergence of resistant bacterial strains, such as methicillin-resistant Staphylococcus aureus (MRSA), the absence of causative organisms following the introduction of community vaccination programs (eg., Haemophilus influenzae type b [Hib]), and the improved ability to isolate specific organisms (eg., Kingella kingae).3) Acute osteomyelitis is approximately twice as common as septic arthritis, and its incidence is steadily increasing.4,5) Up to half of cases have no risk factors.6 However, in the present case, the authors identified trauma as a possible predisposing risk factor for pelvic osteomyelitis. In addition to trauma, several other risk factors have been described (Table 1).7
Table 1 Complications of pelvic osteomyelitis
| Complication |
| Septic arthritis |
| Subperiosteal abscess |
| Myositis |
| Pyomyositis |
| Deep venous thrombosis |
| Permanent impairment (longitudinal growth arrest with subsequent discrepancy in limb length, angular deformity, chronic infection) |
| Pathological fracture |
| Disseminated disease/septicemia |
| Multiorgan failure |
| Death |
Table 2 Risk factors for pelvic osteomyelitis
| Predisposing factors for pelvic osteomyelitis |
| History of recent trauma (open fracture, wounds) |
| Other known concurrent infection (urinary, respiratory) |
| Male sex |
| Age <5 years |
| Retained foreign body |
| Cardiac catheterization |
| Recent surgical procedure |
| Intravascular catheters or other implanted devices |
| Intravenous drug abuse |
Table 3 Differential diagnosis of pelvic osteomyelitis
| Differential diagnosis | |
| Vaso-occlusive disease | Trauma stress fracture |
| Infection septic emboli chronic recurrent osteomyelitis septic arthritis reactive arthritis | Tumor osteoid osteoma acute lymphoblastic leukemia eosinophilic granuloma metastatic neuroblastoma Ewing’s sarcoma osteosarcoma |
| Rheumatologic disease juvenile arthritis | |
Osteomyelitis of the pelvic bone is an uncommon pediatric infection. Delayed diagnosis and treatment can lead to severe complications (Table 2).8) The best diagnostic tool for pelvic osteomyelitis is a high index of suspicion. The most commonly presenting signs and symptoms include fever, pubic tenderness, antalgic gait, and pain with active/passive range of motion of the hip. In fact, the classic scenario of an acutely ill, febrile child with systemic inflammatory signs of septicemia is no longer common.4 The most common clinical picture is similar to the reported case, i.e., a child with fever and gradual progression of local symptoms and signs manifesting as pain and tenderness. Because pelvic osteomyelitis can mimic several different conditions, several differential diagnoses should be considered in the presence of the described signs and symptoms (Table 3).1
Serum markers of inflammation are the most helpful ancillary evaluations for establishing the diagnosis of osteomyelitis. Imaging plays a central role in the diagnosis and management of osteomyelitis. Plain radiography has low sensitivity and specificity for detecting acute osteomyelitis and often shows no abnormalities in the early phase of infection.9,10 In the present case, although pelvic radiography was normal, it was useful to exclude other conditions such as fractures or malignancy. Despite its limitations, plain radiography should remain the first-line imaging modality in suspected osteomyelitis. Ultrasound scan is usually negative, but can be useful in diagnosing adjacent joint septic arthritis and abscesses, which is why it was performed in the present case.7) MRI is the best imaging exam for initial evaluation due to its excellent anatomic detail, high sensitivity for detecting early infection, and lack of ionizing radiation, as shown in the present case.10 It is also helpful in identifying the location and extent of disease, as well as complications in adjacent structures, such as the adductor abscess found in the present case.11
No single test can confirm or exclude pelvic osteomyelitis. Therefore, a combination of careful history and physical examination, accompanied by a high index of clinical suspicion and followed by laboratory and imaging studies, are key components of the clinical investigation.
Staphylococcus aureus is the most common cause of acute osteomyelitis in all age groups. However, a causative pathogen is not identified in up to 55% of cases.11
Treatment principles for pelvic osteomyelitis in children are the same as for other types of osteomyelitis. Therapeutic decision-making is strongly influenced by regional microbiologic epidemiology and antibiotic resistance patterns, with particular focus on the prevalence of local community-acquired MRSA (CA-MRSA). Beyond local microbiodata, the selection of empiric antibiotics requires careful consideration of the patient’s age, clinical stability, suspected site of infection, associated comorbidities, and specific risk factors. Immunization status should be considered, and a second- or third-generation cephalosporin (e.g., cefuroxime or cefotaxime) should be added in children who are not immunized against Haemophilus influenzae serotype b (Hib).1
The 2017 European Society for Pediatric Infectious Diseases (ESPID) guidelines recommend different empirical intravenous antibiotic regimens depending on age. In children aged <3 months, the recommendation is for the combination of cefazolin or an anti-staphylococcal penicillin with gentamicin; in children aged ≥3 months, the indication is for monotherapy with cefazolin or cefuroxime or an anti-staphylococcal penicillin.8
The 2021 Pediatric Infectious Diseases Society (PIDS) and Infectious Disease Society of America (IDSA) guidelines recommend that in regions with a low prevalence of pediatric osteomyelitis-causing CA-MRSA, cefazolin or oxacillin/nafcillin is the preferred empiric therapy for suspected methicillin-susceptible Staphylococcus aureus (MSSA) infection, based on greater safety and tolerability compared with vancomycin or clindamycin and greater efficacy compared with vancomycin. In regions with a CA-MRSA prevalence of 10−20% or greater, clindamycin or vancomycin is preferred. However, cefazolin or oxacillin/nafcillin are still reasonable empiric options for clinically stable children in these regions, pending cultures. In regions where clindamycin resistance of MRSA is substantial (≥10−20%), vancomycin is preferred as empiric therapy for CA-MRSA.12
In the reported case, an empiric regimen of flucloxacillin and ceftriaxone was chosen. The choice of flucloxacillin was based on regional data showing low rates (<10%) of CA-MRSA. Ceftriaxone, which has a long pharmacologic half-life, has a similar bone penetration to penicillins, although its role against MSSA infections remains questionable and its association with flucloxacillin may be dispensable since the current literature recommends empiric monotherapy with flucloxacillin in the present case.12 The inadequate therapeutic response to dual therapy and the presence of local complications prompted the association of clindamycin in an effort to provide optimal empiric therapeutic coverage to CA-MRSA, MSSA, and S. pyogenes. Since agents active against the cell wall may have lower effectiveness in high inoculum disease (eagle effect), or conversely may be effective only in rapidly growing organisms.12,13
The time to switch from intravenous to oral therapy is still a topic under debate, with factors such as apyrexia, pain reduction, clinical improvement, laboratory criteria (reduction in indices such as CRP, erythrocyte sedimentation rate, and leukocyte count), and compliance with oral therapy being investigated.14) The 2017 ESPID guidelines recommend switching to oral therapy after two to four days of IV antibiotic therapy if the patient presents an improvement in clinical conditions: apyrexia or decrease in body temperature for 24-48 h, improvement in symptoms, absence of signs related to complications, 30-50% decrease in CRP compared to the maximum value reached, negative culture tests, and absence of pathogens such as MRSA or PVL-SA (Panton-Valentine leucocidin-positive S. aureus), which can cause more severe forms of osteomyelitis.8,10) However, in the present case, clinical, analytical, and imaging worsening was observed on the day 12, so IV therapy was continued and clindamycin was added.
For children with culture-negative osteomyelitis, the optimal oral agent should have a spectrum of coverage comparable to that of the parenteral agent to which the child showed clinical and laboratory improvement.8) Therefore, in the present case, treatment was changed to flucloxacillin and cefixime in the present case because these agents have a spectrum comparable to that of IV therapy.
Historically, the duration of treatment has ranged from four to six weeks. However, shorter regimens are now recommended for uncomplicated osteomyelitis due to S. aureus in most children.10) In this case, an inadequate initial response associated with complicated osteomyelitis and pelvic involvement motivated prolongation of treatment to a total of six weeks, as recommended in the current literature.8) The recovery rate of pelvic osteomyelitis without sequelae is high (95%), but this rate decreases with delayed diagnosis and treatment failure.10) No sequelae were identified in the present case.
Conclusion
Acute pelvic osteomyelitis in children is rare but curable. It should be considered in the differential diagnosis of any patient experiencing pubic pain aggravated by walking. This case report highlights the difficulty in diagnosing pelvic osteomyelitis. Recognition of the subtle symptoms and signs referred with basic screening tools and early treatment ensures an excellent outcome with negligible long-term sequelae.















