Introduction
Primary lung neoplasms are rare in children, accounting for 0.2% of all malignancies.1,2 Although rare, bronchial neuroendocrine tumors (NETs) are the most common, with an increasing incidence in recent decades (incidence rate of 0.6 cases per million).3
Lung NETs are characterized by neuroendocrine differentiation and can be classified based on histomorphologic features.4 Typical carcinoid tumors account for nearly 80% and are usually low-grade, well-differentiated, slow-growing neoplasms with low malignant potential. Atypical carcinoid tumors have intermediate malignant potential (between typical carcinoids and high-grade small-cell and large-cell lung cancers), exhibit greater mitotic activity and necrosis, more frequently present with hilar or mediastinal nodal involvement, and have a higher recurrence rate. Both tumor types may be positive for neuroendocrine markers.2
Lung carcinoids most commonly arise in the main or lobar bronchi. Typical tumors tend to be more centrally located, whereas atypical tumors are more peripherally located. Common presenting symptoms include recurrent same-lobe pneumonia, persistent cough, hemoptysis, wheezing, and chest pain.1,2,5 The mean age of presentation in children is 12 years, with a slight male predominance.6 Manifestations may be nonspecific, often leading to delayed diagnosis.3 Chest radiograph usually shows nonspecific findings (round or ovoid opacities, hilar masses, atelectasis, and mucoid impaction). Chest computed tomography (CT) with contrast is the preferred imaging modality, and the diagnosis is usually confirmed by bronchoscopic biopsy or transthoracic needle biopsy.2
Resection or ablation can be performed by bronchoscopy.5 However, complete surgical resection is the mainstay of treatment for localized tumors, and systematic nodal dissection should be performed.2,6 Radiation and chemotherapy usually do not play a significant role.1
Atypical carcinoid tumors have a five-year survival rate of 60% compared to 90% for typical carcinoid tumors, regardless of location.5,6 Early detection of well-differentiated NETs has excellent outcomes as surgery can be curative.3 No follow-up is required for node-negative localized completely resected typical carcinoid tumors.2
Case report
A 10-year-old boy with asthma was referred to pediatric pulmonology after eight months of persistent cough, intermittent fever, and dyspnea. Wheezing, hemoptysis, chest pain, night sweats, and weight loss were denied. He had previously been evaluated in an outpatient setting, where decreased right-sided lung breath sounds were noted and he was treated with appropriate oral antibiotics for recurrent right lower lobe pneumonia. Two chest radiographs were obtained showing persistent ground-glass opacities consistent with right inferior lobe atelectasis (Figure 1). CT scan showed a 13 mm intraluminal lesion in the right bronchial tree with contrast enhancement and microcalcifications, adjacent lung parenchyma atelectasis, and mediastinal and right hilar lymphadenopathies (Figure 2). Flexible bronchoscopy revealed a well-circumscribed, round, broad-based mass obstructing the right middle lobe bronchus. Rigid bronchoscopy confirmed a highly vascular neoformation in the apical border of the right lower and middle lobe bronchus. Given these findings, resection of the lesion was performed. Anatomopathological examination showed a solid pattern with nests composed of cells with eosinophilic cytoplasm and round regular nuclei containing finely granular chromatin. The Ki-67 index was 5% with no evidence of necrosis or mitotic figures. Immunohistochemical analysis revealed diffuse expression of chromogranin-a and synaptophysin.
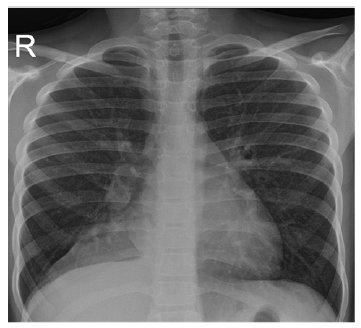
Figure 1 Chest radiograph showing a triangular opacity in the right inferior lobe suggestive of atelectasis.
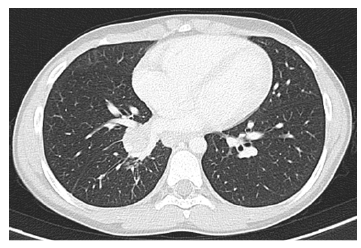
Figure 2 Computed tomography angiography showing an intraluminal lesion in the inferior right bronchus with contrast enhancement and microcalcifications associated with lung parenchyma atelectasis.
Based on the diagnosis of lung NET, the patient was managed by a multidisciplinary team. Gallium68 DOTATOC positron emission tomography (PET) scan showed a nodule in the inferior right bronchus with avid uptake for somatostatin and two right lymphadenopathies and a superior paratracheal lymph node with low avidity, representing local invasion or an inflammatory response. There was no evidence of distant metastases. Serum levels of chromogranin, serotonin, neuron-specific enolase (NSE), and 5-hydroxyindoleacetic acid were normal.
Four months after the first visit to pediatric pneumology, the boy underwent right bilobectomy and regional mediastinal lymphadenectomy through a muscle-sparing thoracotomy. The postoperative period was complicated by a bronchopleural fistula requiring reintervention. Anatomopathological analysis of the bronchial stump confirmed the diagnosis of NET. There was extensive surgical margin involvement without ganglion invasion, and Ki-67 index was 5-10%. Postoperative serum levels of chromogranin-A were normal, but serotonin and NSE were elevated (289 ug/L and 16.55 ng/mL, respectively). Genetic testing excluded germline pathogenic variants in the MEN1 and CDKN1B genes.
During follow-up, the boy continued to have mild exertional dyspnea despite inhaled corticosteroids and physical therapy. Follow-up bronchoscopy showed a well-healed resection site with no evidence of regrowth, and bronchoalveolar cytology was negative for malignant cells. Chest CT scan performed 15 months after surgery showed enlarged right pleuropericardial lymph nodes of suspicious nature and new right paravertebral lymph nodes. PET scan showed no obvious signs of recurrence.
Discussion
In this case, accurate histomorphologic classification was not possible due to incomplete lesion visualization and the presence of crushing artifacts that precluded evaluation of mitotic activity and necrosis. A Ki-67 index greater than 5% was more suggestive of atypical carcinoid. Refinement of small sample diagnosis using biomarkers such as Ki-67 is emerging and appears promising.7
The patient’s history of asthma may have delayed evaluation. The persistent imaging changes raised suspicion of an alternative diagnosis.
Although complete surgical resection is the mainstay of treatment for localized tumors, systematic nodal dissection should be performed, and sometimes lobar or lung resection may be necessary, as in this case.
Considering the inability to make an accurate differential diagnosis between typical and atypical carcinoid and the risk of recurrence (possible locoregional involvement at initial presentation and tumor involvement of surgical margins), close follow-up and long-term surveillance will be required, especially now that the patient is being evaluated for suspected local and regional recurrence.3 Although the follow-up PET scan showed no obvious signs of recurrence, it was decided to repeat chest CT and PET at three and six months, respectively, and to repeat serum levels of the aforementioned tumor markers.
Authorship
Rita Gomes - Conceptualization; Data curation; Investigation; Writing - original draft, Writing - review & editing
Gisela Silva - Writing - review & editing; Supervision
Catarina Carvalho - Writing - review & editing
Fátima Carvalho - Writing - review & editing; Supervision
José Gonçalo Paupério - Writing - review & editing; Supervision
Sílvia Ferreira Silva - Writing - review & editing; Supervision
Telma Barbosa - Conceptualization; Writing - review & editing; Project administration; Supervision















