Introduction
Autoimmune polyglandular syndromes (PAS) are a group of disorders characterized by the association of endocrine and non-endocrine autoimmune features.1) Autoimmune polyglandular syndrome type 2 (PAS-2) is the most common form of PAS. It is a rare disorder in which autoimmune adrenal insufficiency is associated with autoimmune thyroid dysfunction (Schmidt syndrome) and/or type 1 diabetes mellitus (Carpenter syndrome).2,3
Typically diagnosed in adulthood, it is more common in middle-aged women (20-40 years) and has an estimated prevalence of 1.4-2/100,000 inhabitants. There are few reports in pediatric patients.2-4
PAS-2 is associated with genetic abnormalities of human leukocyte antigen (HLA) class II alleles, which regulate antigen presentation to T cell receptors.1,3
The presence of adrenal insufficiency is a clinical criterion for PAS-2. Autoimmune thyroid disease is present in 70% and type 1 diabetes mellitus in 50% of patients.5,6
Adrenal insufficiency is the first endocrine abnormality in approximately 50% of patients, occurs concurrently with autoimmune thyroid disease or diabetes mellitus in about 20%, and after these in about 30%.5,7
Other endocrine disorders may be present in PAS-2, including primary hypogonadism (5-50%) and diabetes insipidus (<1%). In addition, non-endocrine disorders have been reported, although in a much smaller percentage of patients. Vitiligo, myasthenia gravis, celiac disease, pernicious anemia, immunoglobulin A (IgA) deficiency, immune thrombocytopenia purpura, Sjögren´s syndrome, and rheumatoid arthritis are some examples.8,9
Diagnosis is often delayed, sometimes leading to multiple complications. These patients typically present with isolated endocrine dysfunction and later develop other autoimmune diseases, requiring ongoing monitoring.2,3
Case report
A 16-year-old female presented to the Pediatric Emergency Department with a six-month history of asthenia and prostration. Symptoms had started after SARS-CoV-2 infection. She also had a history of nausea for the past three days, recent weight loss, and an episode of vomiting on admission.
Two weeks earlier, the girl had been started on iron (329.7 mg once daily (OD)) and levothyroxine (0.088 mg OD) after being diagnosed with iron deficiency anemia and hypothyroidism by her primary care physician, which she was currently still taking. At that time, she complained of extreme fatigue, but the physical examination was described as normal. The analytical results are shown in Table 1.
The patient denied symptoms such as fever, abdominal pain, anorexia, alopecia, headache, dizziness, bowel dysfunction, polydipsia, or desire to lose weight. She had menarche at the age of 13 with regular catamenia lasting about five days. No significant medical history or regular medications were reported. Family history was positive for thyroid disease, with a grandmother having Hashimoto’s thyroiditis. Other autoimmune diseases were denied.
Vital signs revealed a temperature of 37ºC, heart rate of 109 beats per minute, and blood pressure of 99/58 mmHg (percentile <50th), and the girl was eupneic, with an oxygen saturation (SpO2) of 99% (fraction of inspired oxygen [FiO2] 21%). Capillary blood glucose was 100 mg/dL. At that time, her height was 1.59 m (15th-50th percentile of World Health Organization [WHO] growth charts), her weight was 40.5 kg (loss of approximately 3.5 kg, representing an 8% weight loss in 2 weeks), and her body mass index was 16.1 kg/m2 (3rd percentile of WHO growth charts).
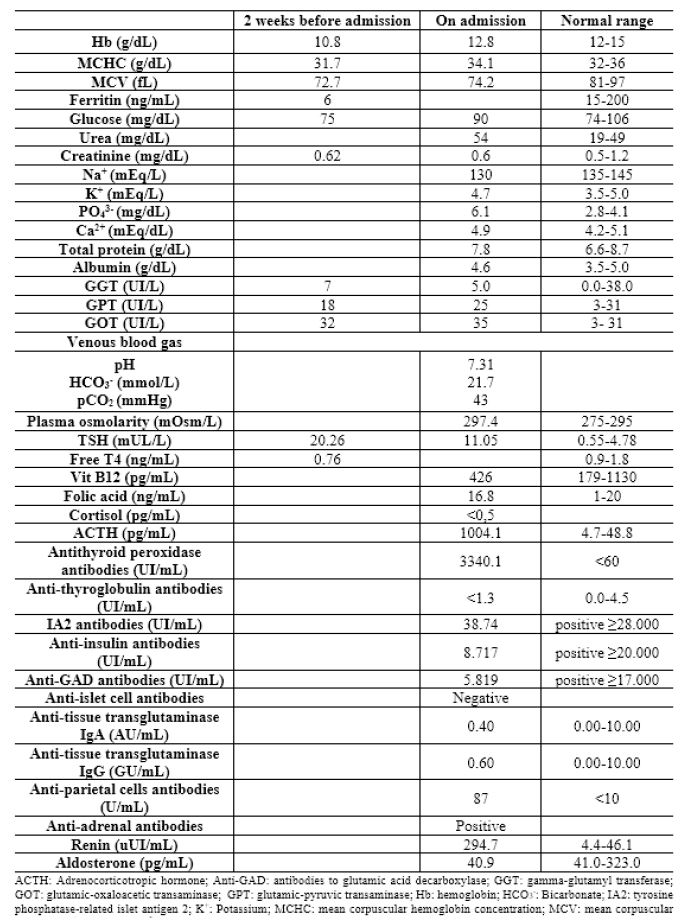
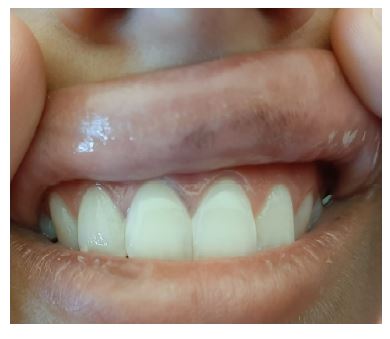
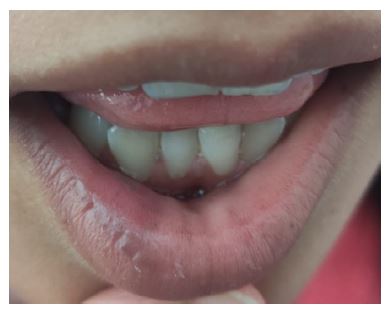
On physical examination on admission, the patient had an emaciated appearance and cold extremities with capillary reperfusion time <2 seconds. She also presented with cutaneous and mucosal hyperpigmentation (Figures 1, 2, and 3). There were no other oral or cutaneous lesions such as candidiasis. The thyroid was not palpable. No other abnormalities were noted.
Laboratory results showed improvement in anemia and a decrease in serum thyroid-stimulating hormone (TSH) levels (Table 1). Urea and plasma osmolarity were slightly elevated. Na+ was low, PO4 3- was high, and venous blood gas showed metabolic acidosis. The remaining results were normal. Ultrasound showed an enlarged and hypoechoic thyroid gland with irregular contours compatible with thyroiditis. Abdominal ultrasound was normal. Hydroelectrolyte and acid-base imbalances improved with intravenous (i.v.) fluids.
Autoimmune Addison's disease related to Hashimoto's thyroiditis associated with PSA-2 was suspected. Treatment with hydrocortisone (i.v. bolus) was initiated and the patient was transferred to a level III hospital for further management.
Subsequent laboratory results showed low cortisol, elevated adrenocorticotropic hormone, and positive anti-adrenal and antithyroid peroxidase antibodies, confirming the diagnosis (Table 1). IA2 and anti-parietal cell antibodies were also positive. Other antibodies were negative. Renin was elevated and aldosterone was slightly low.
During hospitalization, the girl received hydrocortisone (50 mg i.v. every 6 hours [Q6H] for 24 hours [h]), followed by 30 mg i.v. Q6H for 24 h, 20 mg i.v. Q8H for 24 h, and 20 mg per os (PO) Q8H for 24 h). Fludrocortisone (0.1 mg PO OD) was started after 24 h of treatment, and levothyroxine (0.088 mg id) was maintained.
She was discharged after four days of therapy with levothyroxine (0.088 mg id), hydrocortisone (10 mg os Q8H), and fludrocortisone (0.1 mg PO OD).
One month after discharge, the hyperpigmentation had resolved, and the girl had a weight gain of 9 kg. She currently maintains regular follow-up.
Discussion
This case highlights the atypical age of onset and almost simultaneous manifestation of two endocrinopathies: adrenal insufficiency and autoimmune thyroid disease. Hypothyroidism is generally characterized by symptoms such as weight gain, muscle weakness, myalgia, cold intolerance, constipation, and menstrual irregularities. The symptoms of adrenal insufficiency are non-specific and of insidious onset. Weakness, anorexia, fasting hypoglycemia, weight loss, cutaneous and/or mucosal hyperpigmentation, salt craving, nausea, vomiting, and postural hypotension are more evident in intercurrent diseases.
In the present case, the first diagnostic approach to complaints of asthenia in a female adolescent should be to look for anemia or hypothyroidism, as these are the conditions that most commonly justify this symptom.
Two weeks after starting levothyroxine, the adolescent reported worsening symptoms. During this time, she maintained asthenia and prostration and continued to lose weight. She also began to experience nausea and vomiting. All these symptoms raised clinical suspicion of adrenal insufficiency, which was subsequently confirmed.3,10
It has been suggested that treatment with levothyroxine in a patient with unknown adrenal insufficiency may precipitate an adrenal crisis because it increases corticosteroid metabolism in the liver, and this may be what happened in this case.10 Therefore, when the response to hypothyroidism therapy is not as expected, clinicians should be aware of other possible diagnoses, including PAS-2, and treat adrenal insufficiency first.
Recently, some authors have suggested that SARS-CoV-2 infection causes hyperactive immune responses and may trigger autoimmune thyroid disorders. This association was suspected in this case, as the adolescent developed symptoms after SARS-CoV-2 infection. However, further studies are required to confirm this suspicion.8,11,12
Once a second autoimmune disease is detected and PAS-2 is diagnosed, other autoimmune diseases should be investigated. Positive autoantibodies associated with other diseases may be detected months or years before clinical signs or symptoms arise.3,8,10
In this case, in addition to positive anti-adrenal and antithyroid peroxidase antibodies, anti-parietal cell and insulinoma-associated protein 2 antibodies were also detected.
Anti-parietal cell antibodies are present in 85-90% of patients with pernicious anemia, but are nonspecific for this condition, being present in other autoimmune diseases and in some healthy individuals.13 It is acknowledged that pernicious anemia can be associated with PAS-2, but folate and B12 deficiency were excluded in this patient, and microcytic anemia was present.
Insulinoma-associated protein 2 antibodies are a type of antibody responsible for islet cell destruction in some cases of type 1 diabetes mellitus. Although weight loss and asthenia were present in this case, there were no associated blood glucose changes, polydipsia, or polyuria. Therefore, the usefulness of these antibodies in isolation is limited.
Close and long-term follow-up of patients diagnosed with PAS-2 is recommended. In addition, it is important to educate both patients and families about the warning signs/symptoms (e.g., polyuria, polydipsia, polyphagia, etc.) so that not only can the disease be diagnosed at the earliest possible stage, but also life-threatening presentations - such as diabetic ketoacidosis or severe hypothyroidism - can be avoided.3,8,10
Conclusion
This case report illustrates a very rare pediatric condition, PAS-2, in which autoimmune adrenal insufficiency is associated with autoimmune thyroid dysfunction and/or type 1 diabetes mellitus, requiring regular follow-up, appropriate treatment, and early detection of other, not always coexisting, pathologies. Because adrenal crisis can be life-threatening, treatment of adrenal insufficiency should be a priority.
Acknowledgments
The authors acknowledge the patient and family for their permission to publish this case report.
The authors have no potential conflicts of interest relevant to this study.
Authorship
Mariana Vieira da Silva - Conceptualization; Data Curation; Formal Analysis; Resources; Writing - original draft
João Nuno Sousa Marques - Conceptualization; Data Curation; Formal Analysis; Resources; Writing - original draft
Inês Coutinho Oliveira De Lima Madanelo - Data Curation; Formal Analysis; Supervision; Writing- review & editing
Carla Sofia Morgado Pinto Reis - Data Curation; Formal Analysis; Supervision; Writing- review & editing
Joana Filipa Marques Pimenta - Data Curation; Formal Analysis; Supervision; Writing- review & editing
Joaquina Conceição Fernandes Antunes - Data Curation; Formal Analysis; Supervision; Writing- review & editing
Elisabete Maria Costa e Silva Santos - Data Curation; Formal Analysis; Supervision; Writing- review & editing
Cristina Celeste Fernandes de Faria - Data Curation; Formal Analysis; Supervision; Writing- review & editing