Introduction
In recent years, the population of partially dentate adults has increased compared to fully edentulous ones.1-3 According to the 2022 oral health barometer, 48.5% of the Portuguese population has 1 to 8 teeth missing, and 6.4% are completely edentulous.2
Edentulous spaces can be rehabilitated with fixed bridges, implant-supported prostheses, or removable prostheses. A removable partial denture (RPD) with a metal framework is often the simplest and most cost-effective solution for treating patients with multiple edentulous areas.3-8
According to the European Union Medical Device Regulation, removable prostheses are considered medical devices.9 Thus, the ethical and legal guidelines indicate that, in the fabrication of an RPD, the dentist is responsible for planning and prescribing the prosthesis and giving the laboratory all the necessary instructions for its manufacture according to the prescribed design.4-6,8,10 Principles of good practice indicate that fabricating an RPD requires a team approach involving the clinician, the dental technician (DT), and the patient.10
The dentist must inform the DT about the following parameters for RPD construction: the abutment teeth’s periodontal status, the patient’s expectations, and aesthetic parameters. The dentist should also include pre-prosthetic preparations in its planning, which are essential for a successful treatment. In turn, the DT may analyze other parameters in a parallelometer, such as the parallelism of abutment teeth and undercut areas, and transmit them to the dentist.4-7,10
However, the literature shows that the above is not happening in clinical practice. In Ireland, Lynch and Allen reported that 53% of RPD prescriptions received by DTs did not include a design of the metallic structure.3 In Saudi Arabia, Nassani and Alotaibi7 concluded that 64.2% of RPD cases were planned exclusively by the DT. In the United Kingdom, Rice et al.10 observed that 48% of RPD prescriptions did not include occlusal rests, and 30% of the prescriptions that did (51.5%) did not have rest seats in the casts. In Portugal, Caniço5 and Alves6 reported that the DT did not receive design instructions from the dentist in 80% of the cases in Porto and 90.9% of cases in Lisbon, respectively. Thus, although communication between the laboratory and the clinic is essential, it is often insufficient, ambiguous, or even neglected.
This study aimed to evaluate the communication between the dentist and the DT for RPD framework construction by collecting data about fabrication methods and design from Lisbon dental laboratories.
Material and methods
This observational study’s target population was dental laboratories in Lisbon. Written invitations were sent to laboratories that fabricate RPDs in November 2021. All laboratories that voluntarily agreed to participate were included in the study. The study was previously approved by the Ethics Committee of the Faculty of Dental Medicine of the University of Lisbon (October 2021).
Study data were collected through a questionnaire adapted from the one Avrampou et al.4 applied to a Greek population in 2011. The adaptation included adding questions about digital impression and CAD-CAM techniques and removing questions about prosthesis components for RPDs (because the original questionnaire is very long). Three experts (oral health professionals and researchers with experience in questionnaire construction) reviewed the adaptation by verifying the questions’ relevance and clarity. Afterward, a pre-test with one laboratory confirmed the questionnaire’s applicability (December 2021). All laboratories received instructions on how to complete the questionnaire and completed three questionnaires in the presence of the researcher.
The questionnaire included 19 multiple-choice questions about RPD’s design process and fabrication, namely related to the dentists’ prescriptions (2 questions), definitive impression (1 question), conventional impression and its disinfection (5 questions), digital impression (2 questions), cast analysis (2 questions), design (4 questions), and metallic framework (3 questions). A DT filled out a questionnaire for each RPD case with a metal framework after analyzing the dentist’s prescription and the cast. The questionnaire was available for the laboratories between February and April 2022.
Statistical analysis was performed with SPSS version 29.0 (IBM SPSS Statistics, New York, NY, USA). It included descriptive statistics, namely all variables’ absolute and relative frequencies.
Results
Only three of the previously invited laboratories agreed to participate voluntarily. They were established in three parishes of the municipality of Lisbon: Alvalade (lab A), Campolide (lab B), and Olivais (lab C).
After rejecting all questionnaires that were incomplete or had incoherent answers, the sample consisted of 53 entirely completed questionnaires: 16 from lab A, 14 from B, and 23 from C. Each questionnaire was completed based on a single case’s prescription, impression, and cast.
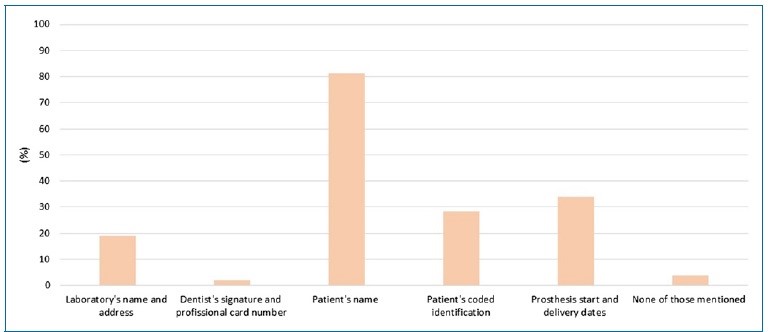
Most prescriptions (62.3%), all in a laboratory form, were sent by general dentists (Table 1). The identifying information in the prescriptions was mostly identification of the patient by name (81.1%), followed by dates of start and delivery of the prosthesis (34%), coded identification of the patient (28.3%), and name and address of the laboratory (18.9%). Only one of the guides had the dentist’s signature and professional card number. Two requests did not include identifying information (Figure 1, Table 2).
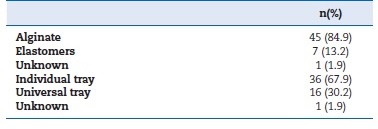
All casts for RPD manufacture were obtained by conventional impression (100%); no case used digital impression. Alginate was the most used impression material (84.9%), followed by elastomers (13.2%). Definitive impressions were made just with a universal tray in 67.9% of the cases (Figure 2, Table 3). All laboratories disinfected every impression received, regardless of having information on prior disinfection in the dental clinic. The prescriptions did not give that information in 20.8% of cases. The material most used by laboratories for disinfecting impressions was a solution of sodium hypochlorite (98.1%) (Figure 3, Table 4).

Figure 2 A) Impression material used for definitive impression; B) Tray used for fabricating removable partial dentures

Figure 3 Disinfection of the impressions: A) information on previous disinfection; B) disinfection in the laboratory; and C) type of disinfection material
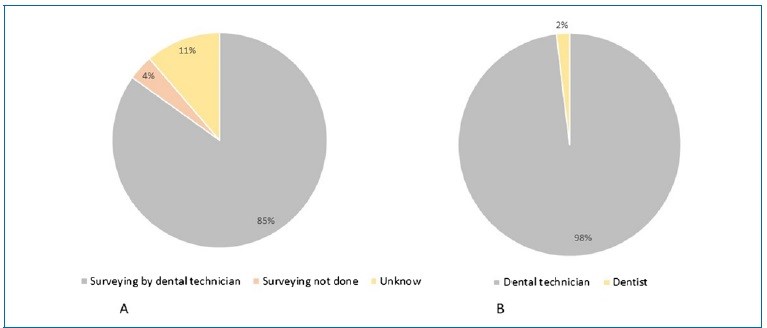
In 84.9% of the cases, the DT analyzed the casts in the parallelometer (Figure 4). Moreover, the DT planned the design for the RPD construction in 98.1% of the cases. The dentist and the DT did not work together in planning the design in any of the cases. In addition to the prescription, the dentist gave the laboratory instructions on the design of the skeletal RPD in five cases, mostly via telephone/mobile phone (7.5%). In one case, the dentist provided a drawing of the RPD structure on paper, with design instructions for the larger connector and the clasps of the metallic structure (Table 5).
Only 34% of the working casts had pre-prosthetic preparations. These cases included preparations for occlusal and cingular rests (34%) and lingual and proximal guide planes (5.7%) (Figure 5, Table 6).
All RPD metallic structures manufactured in the laboratories were produced from cobalt-chromium alloys (Co-Cr), and most were manufactured by electronic induction casting (96.6%).

Figure 5 A) Frequency of pre-prosthetic preparations; B) Pre-prosthetic preparations identified on casts by type
Discussion
Not all laboratories can fabricate Co-Cr RPDs because the necessary equipment implies significant financial costs. Thus, they often forward these works to central laboratories, usually in large cities. Therefore, we analyzed laboratories in Lisbon. Teamwork and communication between the laboratory and the clinic are essential for constructing RPDs. The dentist is responsible for including instructions for the RPD fabrication in the prescriptions,11 as well as the laboratory’s name and address, the dentist’s signature and professional ID number, the patient’s identification by name or code, and the prosthesis’ start and delivery dates.9,11,12 In the present study, none of the prescriptions contained all that information, and two had no information. These alarming data indicate that prostheses are being manufactured without information identifying the patient and the prescriber. Thus, none of the involved professionals can be legally charged if problems arise during RPD’s transport, planning, or manufacture.4-6,8,10 Furthermore, the work might not be delivered, and the patient’s trust in the dentist may be compromised. The failure to send all legally necessary information in practice may result from dentists completing the prescriptions for the laboratory between appointments, with limited time. Given these worrying results, using a digital form that can only be sent once all mandatory fields are completed may be the best solution.
Definitive impressions must reproduce all the anatomical and morphological characteristics of the mucosa and abutment teeth.13,14 They can be obtained by conventional techniques, impression materials, or digital technology.15 Intraoral scanning provides greater comfort for patients with vomiting reflex,15 reduces laboratory time, eliminates possible material distortions,15,16 and allows storing digital data and repeating the prosthesis if necessary.14 Nevertheless, this study’s prescriptions only reported the conventional method. This prevalence may be related to intraoral scanners’ high costs17 and inability to allow functional impressions.15,17 Similarly, Perti et al. reported digital printing use in only 1.39% of the cases.18
Alginate was the most used impression material in the definitive impressions (84.9%). This result agrees with one study in Portugal, in which all definitive impressions were made with alginate,5 and another in England, in which alginate was used in 59% of the impressions.19 Alginate is commonly used because it is easy to handle and less expensive than other available impression materials.13,18 Despite offering poor detail reproduction, it reproduces the teeth and mucosa characteristics required for RPD framework construction.13,19
In this study, only 32.1% of the definitive impressions were obtained with individual trays, which is a low percentage compared to other studies: 82% in Caniço,5 and 60% in Kilfeather.19
Universal trays that are rigid enough and cover all the required structures are suitable for preliminary and definitive impressions.20 However, individual trays are preferable for dental preparations, which are required in RPDs.18
Regarding impression disinfection, the laboratories only had information on previous disinfection in 20.8% of cases and disinfected the impression in 100%, using sodium hypochlorite in 98.1%. The dentist must disinfect the impressions and inform the laboratory accordingly to protect the DT and reduce cross-contamination.5,6,17,21 DTs unaware of previous disinfection may disinfect the impression again - double disinfection - thus reducing detail reproduction and the precision of the impression.21,22,23
Analyzing the study casts in the parallelometer is essential for RPD framework planning.19,24,25 This analysis should be followed by framework design and pre-prosthetic preparations in the oral cavity before the definitive impression.19 In this study, the DT analyzed the cast in the parallelometer in 84.9% of the cases. These results agree with the study by Caniço,5 where the DT did this in all cases. Ideally, the cast should be returned to the dentist after the DT’s parallelometer analysis for the subsequent planning and design of the structure.19
However, although the questionnaire did not specifically ask about it, we assume that did not happen because the RPD framework was designed by the DT in 98.1% of the cases. Pre-prosthetic preparations prevent horizontal loads o the abutment teeth, reduce food impaction, and improve the prostheses’ stability and retention.10,24,25 In this study, 66% of working casts had no prosthetic dental preparation, and only 34% had preparations for occlusal and cingular rests. These data agree with Rice et al.’s study,10 where only 30% of the casts had occlusal rest preparations.
In the present study, the DT planned the RPD design in 98.1% of the cases, which is a very high frequency compared to the literature, namely 54% in Kilfeather’s study.19 These data are alarming because they indicate a lack of instructions and clinical information regarding abutment teeth, which may lead to an inaccurate design of the RPD components, resulting in a dysfunctional RPD.5,6,7,25
The results show that few dentists transmitted information about the RPD design planning, mostly via cell phone/ telephone (7.5%), and only one designed the framework and sent the drawing on paper. Similarly, in Caniço’s study,5 the dentist planned the design in only one case, and in Alves’ study,6 90.9% of the prescriptions had no information about the major connector. Failure to send the design planning may result from a common incorrect practice of delegating the prescription filling to the assistant.
Co-Cr and titanium (Ti) alloys are the most used materials for RPD construction due to their high mechanical strength, high stiffness, and corrosion resistance.26 In the present study, all frameworks were produced using Co-Cr alloys. Similarly, Avrampou4 reported that 88.6% of frameworks were manufactured in Co-Cr alloys. In this investigation, 90.6% of the frameworks were produced by electronic induction casting, and the remaining 9.4% by hand casting. Processing Ti alloys by conventional casting techniques is difficult,27 and no frameworks were produced by CAD/CAM systems using laser sintering in this study. Metallic structures produced with the digital workflow have shown good adaptation, less porosity, and higher precision.28-31
This study had the following limitations: a small sample - only 3 laboratories - and a target population not representative of DTs/dentists in Portugal. It should be interpreted as a preliminar study, and future studies must include a larger sample, more laboratories, and other geographic areas to improve knowledge.
It was perceptible that laboratories found it difficult to take the time to answer the questionnaires, and future investigations should be carried out with smaller questionnaires.