Introduction
Periodontitis is a multifactorial chronic disease characterized by the inflammation of the periodontium. It is accompanied by apical migration of the junctional epithelium, which allows the migration of bacteria along the root surface, leading to the destruction of the connective tissue attachment and alveolar bone loss.1,2 Periodontitis is a high-prevalence disease (45- 50%), and its most severe form affects 10-15% of the adult population.3
Cerebral small vessel disease (CSVD) is a chronic, progressive disorder of arterioles, capillaries, and small veins supplying the white matter and deep structures of gray matter. It is the most common pathological neurological process and the attributable cause of 25% of strokes, besides more than doubling the odds of recurrent strokes. Furthermore, it contributes to 45% of dementia cases and global functional decline.4-6 Abnormalities affecting the structure and function of small vessels of the brain characterize this condition, with numerous neurological and neuroimaging manifestations, of which white matter hyperintensities (WMHs) are the most common.7
The latest evidence seems to suggest that chronic oral infections such as periodontitis contribute to CSVD progression.8 In fact, periodontitis risk factors intersect with those for CSVD and include aging, smoking, obesity, poor oral hygiene, and diabetes mellitus.9-12 Recent studies13,14 found that early tooth loss and periodontal probing depth (PD) were associated with the presence and extension of WMHs.
The aim of this study was to investigate the association between periodontitis and CSVD according to the hypothesis: worse periodontal indicators are associated with a higher load of brain WMHs identifiable by magnetic resonance imaging.
Material and methods
A cross-sectional observational study was conducted in the Blood Pressure Unit of Hospital Pedro Hispano, Matosinhos, Portugal, which is an Excellence Center of the European Society of Hypertension. The local Hospital Ethical Committee approved the study protocol, which was carried out in accordance with the Declaration of Helsinki. All subjects followed the routine clinical procedures and gave written informed consent.
Between 2016 and 2022, a convenience sample of 43 Caucasian patients was included in this study. They were aged between 38 and 82 years, had no previous cardiovascular or cerebrovascular events, and were admitted to the Blood Pressure Unit of Hospital Pedro Hispano, Matosinhos, Portugal.
Patients’ exclusion criteria were significant inflammatory disease, severe brain pathology (dementia by clinical criteria, brain tumor, traumatic brain injury, previous cerebral infection, or neurodegenerative disease), previous cardiovascular events, changes in their ongoing therapy in the last 3 months, pregnancy, tumors, critical illness or a life expectancy under 3 months, and inability to collaborate or to give informed consent.
The following participants’ demographic data were collected either by questionnaire in the first appointment or from existent clinical files: age, gender, height and weight, family history of cardiovascular risk and adverse outcomes,
and calculated body mass index. Clinical data were recorded at baseline and included glycated hemoglobin (HbA1C), fasting plasma glucose, serum creatinine (estimated glomerular function (eGFR) according to MDRD equation), cholesterol (total, HDL, and LDL), triglycerides, ionogram, and uric acid, as well as 24-h urinary sodium and potassium excretion and 24-h controlled urinary creatinine concentration for evaluation of daily salt and potassium intake, as previously described.15,16 Participants’ chronic pharmacological therapies and dietary and physical activity habits were also examined.
Diabetes mellitus was defined by two fasting plasma glucose results ≥ 126 mg/dl, 2-h post-load plasma glucose ≥ 200 mg/ dl, HbA1C ≥ 6.5%, use of antidiabetic agents, or personal history of diabetes.17,18
Information on the periodontal condition was always obtained by the same previously calibrated operator in all presente teeth (six locations per tooth: mesial, central, and distal from the vestibular and palatal/lingual aspects) using a pressure-controlled manual periodontal probe (Hawe Click-Probe® No. 1391, KerrHawe® SA, Bioggio, Switzerland) with a unique click system at a pressure of ca. 20-25 g (0.2-0.25 N), which guaranteed reproducible and precise periodontal assessment.
Two assessments were made with a two-hour interval. If diferences between these two evaluations were found, a third measurement was performed, and the mean between the three was considered. Clinical measurements were then inserted on a periodontal chart (www.perio-tools.com, Department of Periodontology, School of Dental Medicine, University of Bern, Switzerland), and mean probing depth (MPD), mean attachment level (MAL), and bleeding on probing (BOP) were estimated for each patient.
In order to quantify the inflammatory burden posed by periodontitis and its risk factor for other diseases, periodontal epithelial surface area (PESA) and periodontal inflamed surface area (PISA) were also calculated.19 These parameters are further explained below.
Probing depth is the distance from the gingival margin to the apical portion of the gingival pocket and has been used as the main indicator of periodontal inflammation. In our study, it was measured on six locations around each tooth: mesial, central, and distal from the vestibular and palatal/lingual aspects in both arches. The Community Periodontal Index for Treatment Needs (CPITN) for periodontal pocket depth was used with the following criteria to classify periodontitis’ severity: PD of 0-3 mm, no/mild periodontitis; at least one pocket ≥4 mm and <6 mm, moderate periodontitis; at least one pocket ≥6 mm, severe periodontitis.20
The clinical attachment level (CAL) is a parameter that indicates the loss of periodontal support around a tooth, being an indicator of periodontitis severity,21,22 and is related to the distance from the cementoenamel junction (CEJ) to the bottom of the gingival sulcus. BOP refers to the number of bleeding sites relative to the number of probed sites and is one of the key diagnostic parameters for periodontal disease. In this study, BOP was categorized as a dichotomous variable.23 PESA precisely quantifies the total surface area of pocket epithelium. In turn, PISA refers to the area of PESA affected by BOP. PISA’s value, in square millimeters (mm2), represents the degree of periodontal inflammation, thus quantifying the systemic inflammatory burden, which can be used to distinguish subjects regarding their periodontal inflammatory condition.
It is calculated through the association of clinical parameters of periodontal diagnosis such as BOP and Periodontal probing depth, CAL, and gingival recession,19 and is related to parameters of vascular health and low-grade inflammatory systemic markers.24,25 In this study, both PESA and PISA were estimated using the average surface area of each tooth in a freely available Excel spreadsheet.19
Regarding the CSVD-related parameters, WMH volumes normalized by intracranial volume were derived from the T2-weighted fluid-attenuated inversion recovery (FLAIR) sequences collected in the sagittal plane. These FLAIR sequences are much more sensitive to reveal such chronic microvascular damage as hyperintense white matter regions. The following parameters were applied: voxel resolution of 1 x 1 x 1, 256 slices, FOV of 256 mm, TR of 5000 ms, TE of 336 ms, and TI of 1800 ms. Briefly, WMH masks were created using the Lesion Segmentation Algorithm (LPA, 1) from the Lesion Segmentation Toolbox for SPM12 in MATLAB R2018a. Following na initial segmentation of the FLAIR image, probability maps were binarized using the AFNI (2,3, v21.0.15) command 3dcalc. Resulting segmentations were quality-checked for sufficient accuracy, and volumes were calculated using the Freesurfer (v7.1) command mri_segstats. An experienced vascular neurologista evaluated additional signs of CSVD to further characterize the CSVD in the patient cohort. Enlarged perivascular spaces (PVS) were defined as small (<0.3 mm) punctate or linear (if perpendicular or longitudinal to the plane of the scan, respectively) hyperintensities on T2 images in the basal ganglia and centrum semiovale.26
The PVS burden was then stratified into three groups: <11,11-20, and >20.27 Lacunes were defined as rounded or ovoid esions with a diameter >3 mm and <20 mm in the basal ganglia, internal capsule, centrum semiovale, or brainstem, with cerebrospinal fluid density on T2 images.28 Cerebral microbleeds were defined as round, hypodense lesions <10 mm on susceptibility-weighted imaging collected in the axial plane (slice thickness of 2 mm, 256 slices, FOV of 230 mm, TR of 49 ms, and TE of 40 ms), according to the guidelines.29
Most of the continuous variables assumed a non-normal distribution. The association between PD and clinical variables was analyzed considering a significance level (α) of 0.05 and using Pearson’s chi-square and Mann-Whitney rank sum tests.
We then performed generalized linear regression analysis (in univariate analysis, adjusted for significant clinical variables and respective blood pressure mean). Spearman’s correlatio coefficients (Rs) were calculated for the relationship between all variables and PD variables after adjustment.
Results
This study included 43 hypertensive patients, aged between 38 and 82 years (mean 63± 9), of which 39 had their natural dentition (91%), 18 (42%) were female, 21 (50%) were diabetics, and all had previous cardiovascular events. All patients were eligible and agreed to undergo cerebral MRI with Siemens Aera 1.5T (Siemens Healthineers AG®, Erlangen, Germany); data from 6 patients were excluded due to lack of quality for the evaluations.
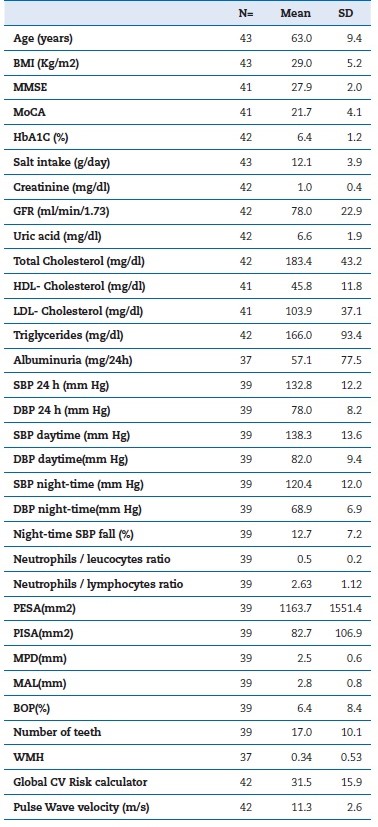
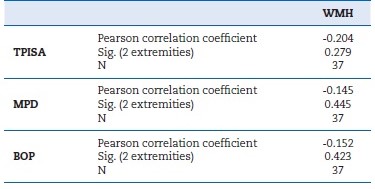
Table 1 shows the descriptive analysis of the study sample. All patients were under anti-hypertensive drugs, 52% were under statins, and diabetic patients were all under oral antidiabetic medication. The MPD of the sample was 2.5 mm, the mean PISA was 82.7 mm2, the MAL was 2.8 mm, and the mean BOP was 6.4%. Table 2 shows the correlations between total periodontal inflamed surface area (TPISA), MPD, BOP, and WMHs, and Table 3 shows the correlations between MAL and WMHs (α=0.05).
Table 2 Correlations between total periodontal inflamed surface area (TPISA), mean probing depth (MPD), bleeding on probing (BOP), and white matter hyperintensities (WMH) (α=0.05).
Discussion
Surprisingly, data analysis revealed that an increase in WMHs identified by MRI was associated with fewer periodontal inflamed areas, reduced pocket PDs, and lower BOP percentages.
These findings might be associated with the nature of the periodontal indicators used, even though these results contradict those previously described in other studies. Still, those studies with which we compared our results employed diferente methods susceptible to unfair outcomes. For example, when investigating the association between tooth loss and brain white matter changes, Minn et al.13 considered patients who had lost 1 to 5 teeth as the control group, and only patients with 6 or more lost teeth as periodontally compromised, even though no periodontal examination was done.
This methodology arouses several doubts regarding the genuine periodontal condition of the study participants. Nevertheless, assuming that the lower the residual amount of supporting bone, the higher the probability of tooth loss,30 the results obtained by Minn et al.13 agree with our study. Another critical difference concerning the methodology used that may impact the results obtained is that brain white matter changes were diagnosed by computerized tomography scan rather than magnetic resonance image, which we know has higher sensitivity and specificity for detecting pathological changes.28
Regarding MAL, results suggest a positive association with WMHs, although not statistically significant. We believe this lack of statistical significance is related to the reduced sample size in our study. Nevertheless, we consider relevant that the attachment level, as a determinant of the accumulated periodontal destruction, is associated with more WMHs. Contrarily, TPISA, MPD, and BOP, all of which represent the periodontal condition at the moment, are not as representative as the attachment level regarding the cumulative nature of the brain white matter degeneration.
Hada et al.14 studied the relationship between periodontal condition and WMHs in an adult Japanese population. Magnetic resonance imaging was used to identify brain white matter changes. The patients’ periodontal status was obtained by measuring PD in 10 teeth per patient, regardless of the number of existing teeth, as follows: at least one PD of ≥ 4 mm was classified as moderate periodontitis, and one or more PDs ≥ 6mm was classified as severe periodontitis. Results revealed a statistically significant association between WMHs and severe periodontitis, and no significance for moderate periodontitis.
Thus, the authors concluded that severe periodontitis might influence the risk of developing WMHs. Contrarily to Hada et al.’s14 findings, our data showed an inverse association, though not statistically significant: more WMHs were associated with lower values of PDs. This outcome may result not only from the sample’s small size but also because PD only describes the periodontal condition at the moment and, so, is not a trustworthy indicator of the cumulative nature of periodontitis and, consequently, of its association with WMHs. Mayer et al.,31 more recently, aimed to establish the association between periodontal disease and microstructural brain alterations in the Hamburg City Health Study (HCHS).
Once the attachment loss was determined, all study participants were submitted to 3-T magnetic resonance imaging.
The results, adjusted to gender and age, showed that all periodontal indicators were significantly associated with more WMHs, and, mainly, the higher the attachment loss, the higher the number of white matter lesions. On the other hand, when the statistical model was adjusted to education and cardiovascular risk, only plaque index remained significantly associated with WMHs, and attachment loss was positively associated without statistical significance, as reported in our study. In Mayer et al.’s study,31 the WMH quantification was more precise because their methodology enabled identifying subtle brain microstructural changes well before these produced visible vascular damage by conventional magnetic resonance image (1.5T). Thus, that study identified brain white matter lesions that would not have been identified with standard magnetic resonance imaging, which means WMHs were overvalued. Chronic inflammation plays an important role in the development of CSVD because of its nefarious effects on healthy tissues. Once inflammatory mediators reach endotelial cells, they can cause the rupture of the brain-blood barrier32-34 - the mechanism underneath the pathogenesis of the disease since it facilitates the transit of harmful substances to brain tissues, causing vascular changes.35 Therefore, plausibly, because periodontitis is one of the most common chronic infections of the oral cavity, it assumes a key role in the association with CSVD. Periodontitis’ inherent chronicity and complexity, along with the persistent inflammatory reaction transmitted to the teeth’s supportive tissues and consequente discharge of numerous inflammatory mediators, lead to endothelial dysfunction, playing a critical role in the pathogenesis of CSVD.
The fact that periodontal treatment enhances endotelial function strengthens the linkage between these diseases,36 and therefore, periodontal patients should have an increase risk for CSVD.37 Due to anatomic proximity between the mouth and the brain, bacteria, such as Porphyromonas gingivalis, found in gingivitis and periodontitis, can travel through the bloodstream and reach the cerebral tissue. Corroborating this theory, a study by Lee et al.38 showed that periodontal treatment and dental prophylaxis toward Porphyromonas gingivalis can prevent ischemic stroke. Also, studies have demonstrated an association between the presence of Porphyromonas gingivalis and an increased risk of ischemic,39 cardioembolic, and thrombotic stroke.40
This study has some limitations, such as enrolling a small population within a narrow age limit and a single location. All participants were hypertensive, with high cardiovascular risk, and under several medications, so the generalization of the results to other populations is obviously limited. Since it is a cross-sectional observational study, we cannot affirm the causality between periodontal disease and WMHs. Therefore, large prospective studies are needed to enhance the current evidence regarding the association between periodontal disease and CSVD.
Conclusions
In our study, periodontal PD, BOP, and total PISA were negatively associated with brain lesions identifiable by magnetic resonance. WMHs were correlated with a determinant of periodontal disease, which is the more accurate indicator of the periodontal support around a tooth. Nevertheless, it remains unclear whether the relationship found between WMHs and periodontal disease is purely circumstantial because of the simple coexistence of highly prevalent phenomena. Thus, the possible causal relationship between periodontal disease and cerebrovascular disease remains unclear.