Paclitaxel: background
Taxol, precursor name of paclitaxel, was initially discovered in 1964, and first extracted from Taxus brevifolia tree. After extensive research, commercialization started in 1991 and in 1994 was approved by the FDA for the treatment of ovarian cancer. Paclitaxel interferes with the normal function of microtubules, arresting them by hyper-stabilizing their structure, and destroying the cell's ability to use its cytoskeleton in a flexible manner. Further research has indicated that paclitaxel induces programmed cell death (apoptosis) in cancer cells by binding to an apoptosis stopping protein called Bcl-2 (B-cell leukemia 2) and thus arresting its function.1,2 Therefore, it has an antiproliferative function. Its metabolism is mainly hepatic (90%) and 10% of excretion is kidney mediated. After IV infusion, elimination half-lives of paclitaxel have been reported from 6-12h.3 Paclitaxel has obtained FDA approval for the treatment of multiple neoplasms, including breast, ovarian and lung cancer, and also Kaposi's sarcoma.3-5 More recently, they have been approved has a component of endovascular devices for the treatment of coronary or peripheral artery disease.
Paclitaxel in peripheral angioplasty devices
Vascular wall restenosis is a proliferative event, at least in its early stages. Researchers reasoned that paclitaxel could potentially inhibit multiple processes involved in the development of restenosis after open and endovascular intervention. Biologically, can be divided in three phases: acute vessel recoil after angioplasty; after this initial injury vessel wall myofibroblasts are stimulated to produce extracellular matrix and subendothelial exposure activates platelets and results in local inflammation; this inflammation results in migration of vascular smooth cells and fibroblasts into the area of injury. Paclitaxel was shown in animal models to exhibit a dose-dependent inhibition of smooth muscle cell proliferation and migration by irreversibly stabilizing intracellular microtubules, at concentrations up to 100 times lower than antineoplastic levels. Because smooth muscle cells are more sensitive than endothelium to paclitaxel, it was proposed that it could inhibit restenosis with minimal damage to the arterial wall.6 With that in mind, first human use of paclitaxel was in the coronary circulation to prevent or to treat restenosis, and subsequently its use was extended to the peripheral arteries.
Paclitaxel-coated devices (PCD), that comprise drug-coated balloons (DCB) and drug-eluting stents (DES) became commercially available for the treatment of peripheral artery disease in 2015 and 2012, respectively. Currently, there are multiple brands producing coated balloons and stents that can be used in almost every peripheral vessel.7
These devices have been specifically engineered to deliver prolonged levels of paclitaxel into the vessel wall in order to inhibit smooth muscle cell proliferation and avoid hemodynamically significant vessel restenosis. To enable sustained tissue bioavailability, most modern balloons and stents are coated with a mostly solid crystalline form of paclitaxel. Some studies demonstrated local therapeutic paclitaxel levels up to 2 months after delivery. However, almost 90% of the drug delivered is lost to systemic circulation, with only 5-10% actually being transferred to the vessel wall.8,9
Long-term results of paclitaxel coated-devices
Multiple clinical trials have demonstrated the superiority of PCD relative to bare-metal stenting (BMS) and plain-old balloon angioplasty (POBA) in terms of primary vessel patency, need for target lesion revascularization and late-lumen loss. The THUNDER Trial evaluated late-lumen loss in 154 patients with femoro-popliteal lesions. At 6 months, they reported significantly less late-lumen loss in the PCD group relative to those treated with POBA (0.4 ±1.2 vs. 1.7 ±1.8 mm , p<0.001) and significantly lower rates of TLR in the PCD group at 6 and 24 months (4% vs. 37%, p<0.001 at 6 months and 15% vs. 52% at 24 months, p<0.001). At 5-year follow-up, the PCD group had significantly lower rates of TLR and binary restenosis compared with control (21% vs. 56%, p=0.0005 and 17% vs 54%, p=0.04), respectively.10
In the IN.PACT SFA trial, 331 patients with femoro-popliteal lesions were randomly assigned to receive a DCB or uncoated device. At 1-year DCB resulted in higher primary patency versus PTA (82.2% versus 52.4%; P<0.001). The rate of clinically driven target lesion revascularization was 2.4% in the DCB arm in comparison with 20.6% in the PTA arm (p<0.001).
At 2 years, primary patency of DCB was also higher when compared to PTA (78.9% vs. 50.1%; p < 0.001). The rates of TLR were 9.1% vs. 28.3% (p < 0.001) for the DCB and PTA groups, respectively. At 5 years of follow-up, patients treated with DCB had greater freedom from TLR relative to those treated with PTA (74.5% vs. 65.3%, p=0.02).11
Table 1 Summary of long-term patency outcomes of PCD for the treatment of femoropopliteal disease
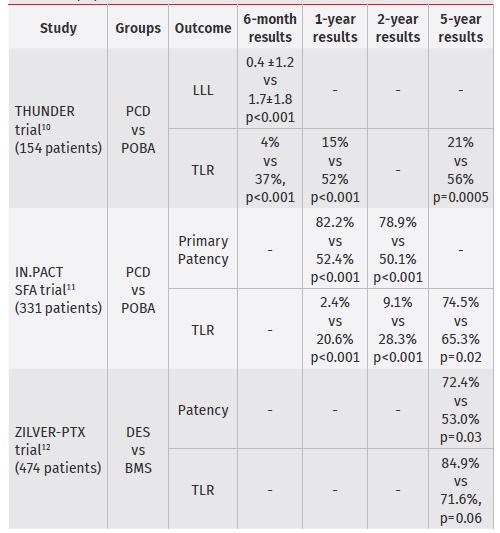
PCD - paclitaxel-coated devices; POBA - plain-old ballon angioplasty; DES - drug-elluting stent; BMS - bare-metal stent; LLL - late-lumen loss; TLR - target-lesion revascularization
Paclitaxel-coated stents have also demonstrated benefit over uncoated devices. In the Zilver PTX trial, comprising 474 patients, clinical benefit (persistent or worsening symptoms of ischemia; 81.8% vs. 63.8%, p = 0.02), patency (72.4% vs. 53.0%, p = 0.03), and freedom from TLR (84.9% vs. 71.6%, p = 0.06) were significantly higher at 5 years of follow-up for DES relative to BMS.12
Risk of death of paclitaxel coated devices in femoro-popliteal arteries
Since the end of 2018, concerns about safety of PCD have been raised, concerning late all-cause mortality, specifically in two meta-analyses, by Katsanos et al and Rocha Singh et al.9,12 These findings had global implications, temporary halting enrolment in 2 RCT and in international guidelines.13 Katsanos et al meta-analysis included 28 RCT with a total of 4663 patients and a median follow-up period of 2 years (1-5 years).
All-cause mortality was assessed at 1, 2 and 5 years after index procedure. For a total of 4432 subjects, all-cause death at 1 year was not significantly different between groups (2.3% vs 2.3%; RR 1.08 - 95% CI .72-1.61). At 2 years, 12 RCT analysis revealed a significantly increased risk of death in patients exposed to paclitaxel (3.8% vs 7.2%; RR 1.68 - 95%, CI 1.15-2.47; NNH 29). At 5 years, 3 RCT (863 patients) were included, and paclitaxel exposure was associated with a significant risk of death (8.1% vs 14.7%; RR 1.93 CI 1.27-2.93; NNH 14). Rocha Singh et al., on an individual patient data meta-analysis of the same patients, using individual data of patients from US manufacturers PCD, found an excess relative risk of 27% in patients treated with paclitaxel.
Since initial concern about safety of paclitaxel coated devices has been raised, multiple observational cohort studies have been designed. Bertges et al in a 8376 patient cohort, found a benefit in mortality rates in paclitaxel-exposed patients at 1 year (11.5% vs 8.5%; HR=.82; 95% CI .68-.98, p=.03).14 Secemsky et al, in a 16650 patients cohort, found no association with paclitaxel exposure and all-cause mortality through 600 days post-procedure (34.3% vs 32.5% for control and paclitaxel exposed groups, respectively; p=.007).15
Freisinger et al for a total of 64711 patients from a health insurance database, found a tendency for increased mortality with DES after the fourth year, however not statistically significant for up to 11 years (HR between .64 and 1.10; all p>.057). For DCB, there was a decreased mortality in the first year (HR .92; p< 0.001), which became irrelevant in the following years.16
Behrent et al, using the same database from 2010 to 2018, including 21546 patients, found that paclitaxel exposure was associated with improved survival (HR 0.83, CI 0.77-0.90)17
Katsanos et al have postulated that late paclitaxel toxicity may be the reason for the observed increased death rate. That is justified by the long half-life (up to months) of paclitaxel crystals deployed on the vessel wall, contrary to the short half-life of paclitaxel after IV infusion. When applying PCD, just 10% of paclitaxel deployed is absorved by the vessel wall, and as much as 90% is lost to the systemic circulation. This as the risk of systemic side effects, despite the lower doses when compared to the chemotherapy formulation, and a non-dose dependent effect is plausible. Beyond a prolonged half-life, paclitaxel crystals improve tissue uptake of the drug, enhancing both the potencial benefit as the risks. As the vessel uptake of crystals is low, there is a worrisome of microparticle formation with the risk of distal embolization. This is postulated to be responsible for the significantly higher rates of major amputations noted in the active paclitaxel arm of the INPACT-DEEP study.8,9
Conclusions and what should the patients know
When using paclitaxel coated devices, several issues should be kept in mind due to late mortality trend found in Katsanos et al. study. Firstly, results were based on summary-level trial data, which cannot account for data at the individual patient level. This limitation was, however, surpassed by Rocha-Singh et al, who still found an excessive risk of death in paclitaxel exposed patients. Secondly, many of the RCTs analyzed were not specifically powered for this late outcome analysis. Thirdly, the large majority of patients analyzed were claudicants. Conversely, in Portugal, CLTI is the most frequent PAD-related cause of hospital admission.18
It should be noted that CLTI patients correspond to a more serious subset of PAD patients, which are older, frailer, with more severe comorbidities and with more advanced and diffuse atherosclerotic disease. In these patients, the inbalance between limb and quality of life outcomes versus a possible increase in late mortality should reflect these intrinsic differences. The differences between results of RCT meta-analyses and cohort studies must be present. The higher mortality risk in “controlled scenarios” was not verified in “real-life settings”, namely in cohorts with a superior prevalence of CLTI and follow-up periods up to 11 years presenting with pointwise benefit in mortality rates.17 However, these data do not undoubtedly show a mortality benefit for coated devices and do not invalidate the findings of Katsanos et al.9
Table 2 Risk of death of paclitaxel coated devices in femoro-popliteal arteries
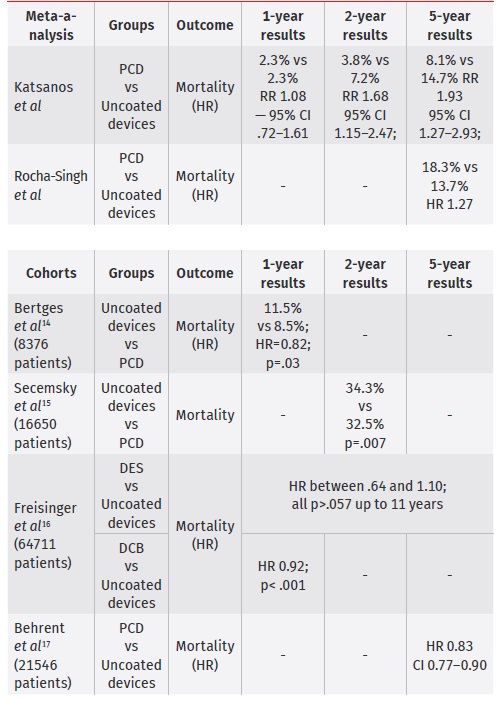
HR - Hazard Ratio; RR - risk ratio DES - drug-elluting stent; DCB - drug-coated balloon
Informing patients of the relative risks and benefits of paclitaxel devices is critically important, as this is an intrinsic part of a healthcare practitioner duty.
This process also requires considering patient age, comorbidities, life expectancy, risk factor control, clinical presentation (claudication vs CLTI) and his expectations and wishes concerning limb outcomes. They also must be informed of the lower patency and higher need for reintervention after treatment with uncoated devices. A brochure with the reported risks and benefits may be designed. This should be always an active and shared process. Patients must be advised of how many paclitaxel coated devices may be used for a successful procedure and informed of other therapeutic options (medical treatment exclusively, open revascularization, endovascular revascularization with uncoated devices or even major amputation). After all this process, an informed consent that specifically specifies consent to use paclitaxel coated devices might be obtained. Patient records should specifically specify what, where and how many devices were used. This is not exclusive for coated devices, ideally all patients should have some registry that explicitly reports the implanted devices, as this may reveal important for things as simple as to know if the device is MRI compatible. Follow-up adjustments could also be advised until this matter is clarified. However, since the specific mortality causes related to paclitaxel exposure were not known and a prophylactic measure to reduce late mortality is missing, a desirable closer follow-up may be futile and fruitless.7
The authors believe that adequately powered RCT that evaluate short and long-term impact on mortality, health and limb related quality of life in either claudicants or CLTI patients should be pursued. In claudicants, a group with higher life-expectancy, is critical to know if the mortality risk surpass the benefit in quality of life. In CLTI patients, we need to understand the long-term results who are underrepresented in RCT. Lastly the late mortality trend, mainly in claudicants patients, with no described mechanism, should lead to a reconsideration of future trial designs.














