INTRODUCTION
Basal cell carcinoma (BCC) represents the most common human neoplasm in Caucasians, corresponding to about 80% of nonmelanoma skin tumors.1
It is a malignant epidermal tumor, locally invasive, slow-growing, and whose incidence of metastases is greatly reduced, ranging from 0.0001% to 0.55%.2
Most metastatic BCC’s originate from the head and neck, with the bone being involved in approximately 20% to 30% of cases, since me-tastatic dissemination occurs more frequently to the regional lymph nodes (60%) and the lung (40%).3
Although, surgery represents the most successful treatment for the majority of BCC’s, there is no effective therapy for locally advanced or metastatic disease. The average survival is only 8 - 10 months.4,5
New molecular therapies, such as anti-epidermal growth factor re-ceptor drugs and inhibitors of the Hedgehog (Hh) pathway, are emerging as more promising alternatives for patients with metastatic BCC.6
We present the case of a young adult with cutaneous BCC of the face with an atypically extensive bone metastases development, aiming to improve the management of this type of tumor and to highlig-th some of the latest trends in molecular therapy for advanced BCC.
CLINICAL CASE
In November 2011, a 29-year-old male patient with prolonged smoking history (14 pack-years) and no relevant previous medical history, namely primary/acquired immunodeficiency, prior radiation therapy, or knowledge about any genodermatosis with oncogenic predisposition (e.g., Gorlin syndrome), was diagnosed with morpheaform BCC, after excision of an exophytic lesion located in the right genian region. He showed no other lesions or palpable cervical masses.
Subsequently, he underwent two surgical excisions, with com-prehensive intraoperative margin control, due to local recurrences: the first, in December 2012 and the other in May 2015, were both incomplete. The histopathological examination of the latter revealed a BCC lesion with 1.8 cm of extension and 3 to 4 mm of thickness, of infiltrative morphology, aggressive behavior, perineural invasion and deep (with muscle invasion) and internal margin envolvment.
Surgical margins were enlarged (with excision of previous scar), which proved to be free of neoplastic tissue.
In June 2015, the patient had a reconstruction of the right hemiface with cervical-thoracic flap, with adequate scar evolution and satisfactory aesthetic result. Clinically, he had no pain, although he evidenced sensory hypoesthesia at the right hemiface level.
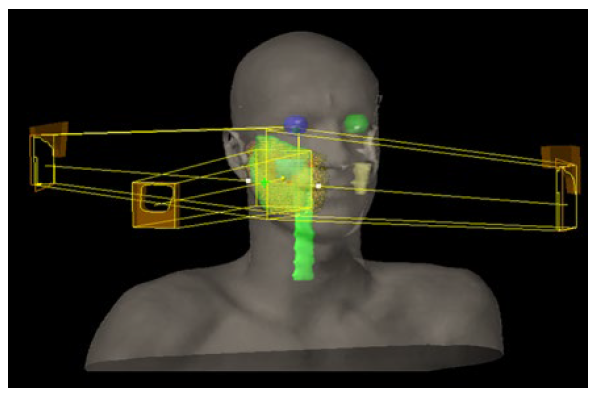
Between August and September 2015, the patient received adjuvant three-dimensional conformal radiotherapy (3D-CRT), with 50 Gy in 25 fractions, directed to the right hemiface, followed by a boost of 10 Gy in 5 fractions to the tumor bed (Fig. 1), without significant complications.
The patient remained under clinical surveillance, with no eviden-ce of locoregional disease and recovery of facial sensitivity.
By the end of 2016, due to low back pain, the patient performed a computed tomography (CT) scan of the lumbosacral spine that showed bone changes in the body of S1 and in the right wing of he sacrum. On May 2017, a biopsy of the sacral lesion revealed a morpheaform BCC bone metastasis.
The restaging 18F-fluorodeoxyglucose (18F-FDG) PET-CT showed extensive lytic bone metastases.
The patient started vismodegib (150 mg, once daily) in June 2017 and, in parallel, he was treated with 3D-CRT to a total dose of 16 Gy in 4 fractions directed to segments D7-D10 and the posterior segment of the 8th rib, with antialgic palliative purpose.
In the next two months, the patient presented considerable impro-vement of pain, and maintained supportive analgesic therapy.
In September 2017, the reassessment PET-CT revealed a heterogeneous response to vismodegib, with reduced metabolic activity of existing lesions and appearance of new lesions. In October 2017, the patient was admitted for symptomatic control of severe pain at the lumbosacral spine, refractory to analgesic therapy. In this context, segments L3-S3 were irradiated with 16 Gy in 4 fractions.
In November 2017, due to pain recurrence at the lumbar region, reirradiation was performed to the L5-S3 segments, with 30 Gy in 10 fractions.
Due to a weak response to vismodegib, systemic therapy was chan-ged by adding pembrolizumab (200 mg, each three weeks) in December 2017, and upon disease progression in the PET-CT, it was decided to suspend pembrolizumab in April 2018, and then start chemotherapy with cisplatin and 5-fluorouracil (six cycles, with partial, heterogeneous and symptomatic improvement). In May 2018, the patient also began denosumab (120 mg, once a month).
Subsequently, an incisional biopsy of a new centimetric nodular cutaneous lesion in the right arm revealed an infiltrative BCC metastasis (Fig.s 2a-b). The immunohistochemical staining showed that tumor cells were positive for CK5/6, p63, Ber-EP4 (Fig. 2c), and negative for CK20, thus supporting the diagnosis.
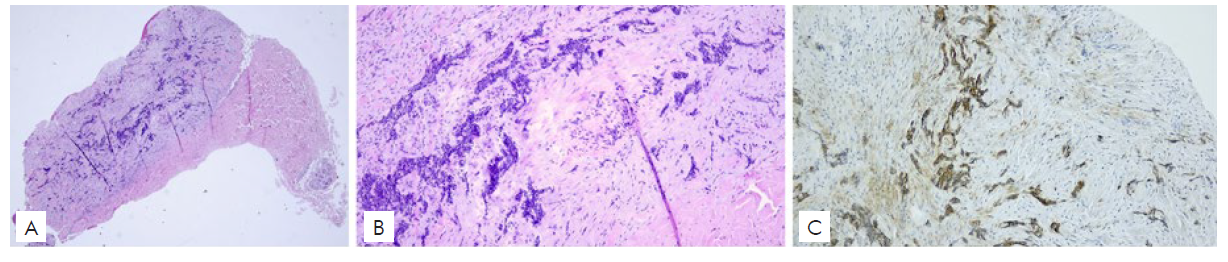
Figura 2 Histological images of the cutaneous BCC metastasis (Haematoxylin and Eosin) 100x (a), 400x (b), demonstrating typical basaloid morphology, and immunohistochemistry for Ber-EP4 with intense positivity of the neoplastic cells (c).
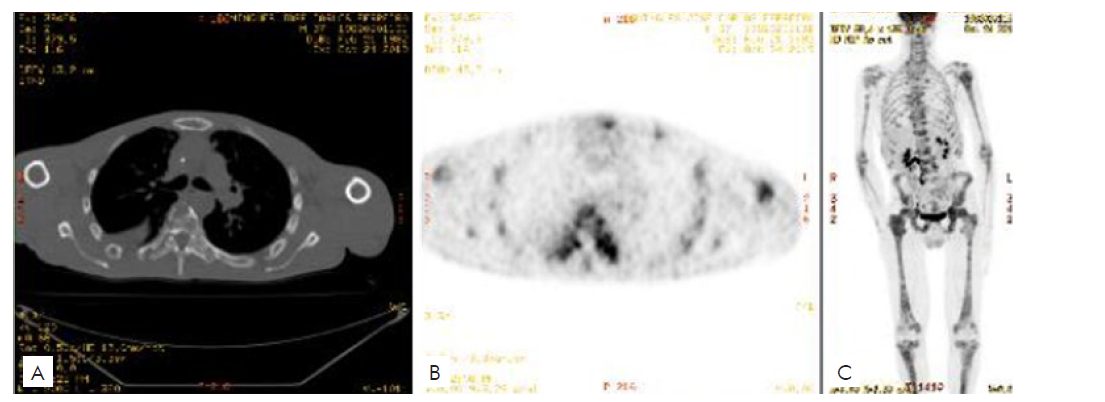
PET-CT performed in June 2019 revealed diffuse spreading bone metastases, the appearance of new cutaneous and subcutaneous metastases in the frontal region, and bilateral pulmonary micronodulation. The patient was then recommended to for a second line of chemotherapy with carboplatin and paclitaxel. However, in October 2019, PET-CT noticed the massive progression of bone and lung metastases (Fig. 3), and so it was decided to do a therapeutic switch to itraconazole (800 mg, once daily), discontinued three months later, due to poor response and adverse events, mainly fatigue.
By this time, the patient developed horizontal diplopia, with apparent paresia of the VI right craneal nerve. A magnetic ressonance imaging of the skull showed metastatic infiltration of the clivus bilaterally, as well as an expansive lesion at the base of the left orbit.
Since November 2019, the patient maintained best supportive care, and died on December 23, 2019 - about 3 years after the diagnosis of metastatic disease and 8 years after the onset of the primary lesion.
DISCUSSION
Although typically indolent, BCC can rarely metastasize at distance, considerably decreasing survival (10% at 5 years).7
Based on published reports between 1981 and 2011, McCusker et al8 provided a review of 100 patients with advanced BCC, which revealed that distant metastases were diagnosed at an younger age (mean 58 years versus 66.3 years for regional metastases) and were associated with shorter overall survival time since diagnosis (24 versus 87 months). Bone metastases have been reported in 24% of patients with a median survival of 12 months (versus 26 months in patients without bone metastases). The McCusker et al review also mentions that the most common histological subtypes in primary tumors were, by this order of frequency, infiltrative, metatypical (basosquamous), and morpheaform.
Certain clinical and histopathological characteristics of BCC have been considered relevant for its probability to metastasize7,8and are recognized in this patient: male gender; young age of onset; location of the primary tumor on the head; short time interval between the onset of primary lesion and development of bone metastases; incomplete initial excision, with immediate healing; resistance to treatment.
Another contributing factor for distant metastases is local recurrence after surgical excision, especially when it is impossible to obtain free margins, which may result from the size and extent of the tumor.9
In the series of six cases of metastatic BCC, described by Lau et al,10 the most common histological subtypes were morpheaform and infiltrative, which present an aggressive growth pattern. This fact was added to the presence of perineural and/or lymphovascular invasion in two of them, the first aspect also found in this case.
A curious fact in this case, is that there was no local recurrence of the primary lesion, after its reexcision and adjuvant external radiotherapy, which was also found in five of the six patients in the series of Lau et al.10
Post-operative external radiotherapy is associated with reduced risk of recurrence in high-risk patients, with local control rates of around 100%,11 and is recommended after incomplete ressection with microscopic (R1) or macroscopic (R2) residual tumour, if clear margins cannot be achieved, and also in patients with negative margins but evidence of considerable perineural involvement.
It should be noted that external radiotherapy represents a globally safe and well tolerated procedure, with improved cosmetic results.
Combination therapy between various modalities (chemotherapy, targeted therapy, immunotherapy), in addition to external radiotherapy, is currently the standard of care in metastatic BCC.12
Recent developments on the immunological treatment of BCC have shown that inadequate signaling in the Hh pathway contributes significantly to the pathogenesis of a large percentage of localised and metastatic BCC.6
However, only two such Hh pathway inhibitors, vismodegib and sonidegib, are currently approved as systemic therapies for locally advanced and metastatic BCC.13,14
In this case, treatment recommended by the multidisciplinary team was vismodegib, an oral antagonist of the smoothened (SMO) protein involved in the Hh signaling pathway, investigated in a randomized phase II trial (ERIVANCE),13 which showed a response rate of 30% and a median response duration of 7.6 months, in 33 patients with metastatic BCC. Clinical investigation into the efficacy of these therapeutic agents is still ongoing, with response rates ranging from 15% to 48.5% in advanced BCC.15-17
There is evidence that resistance to vismodegib may arise from mu-tations of the SMO protein or from downstream changes in the Hh pathway,18 which could be an explanatory hypothesis in this case.
Furthermore, other pathways, such as the Wnt/β-catenin, interact with the Hh pathway. Therefore, investigating the cross-talk between those two signaling pathways, could be valuable in identifying better therapeutic strategies for advanced, inoperable and/or resistant BCC’s.19
Chang et al20 suggest that due to high levels of programmed death ligand-1 (PD-L1) expression in BCC, programmed death protein-1 (PD-1) inhibitors, such as pembrolizumab, may be a useful therapeutic option against advanced disease, especially when BCC is refractory to Hh pathway inhibitors.18
Unfortunately, in this patient treatment with anti-PD-1 pembrolizumab did not restrain BCC progression, which became an additional treatment challenge, since the mechanism of resistance by the bone metastasis to PD-1 inhibition is unclear and under ongoing investigation.18
Given the absence of an objective response, treatment with those targeted agents was discontinued and further management with chemotherapy was chosen. Due to the rarity of metastatic disease, information regarding chemotherapy is scarce, with reports on the use of platinum-based agents21 and favorable responses described in 38% of the patients.8
Other known molecules, such as itraconazole, especially in cases of resistance to SMO inhibitors, have shown efficacy, as demonstrated in an open-label, exploratory phase II trial.22However, our patient did not respond to itraconazole, which confirms the need for more studies and long-term follow-up for appropriate clinical evaluation in patients with refractory metastatic BCC.
CONCLUSION
BCC is a very common tumor and the presence of metastases, al-though rare, should not be disregarded, as it implies high morbidity and mortality.
Due to the treatment and prognostic implications of metastatic BCC, it is essential to identify risk factors, in order to allow tighter sur-veillance of high-risk patients, with close clinical monitoring and serial imaging, and early implementation of adequate therapeutic strategies.
Follow-up of patients with BCC should include regular physical exams, including skin self-examination (6 - 12 months), particularly during the first two years, from which the frequency of the same can be reduced. A tighter and longer-term surveillance (for 3 - 5 years) should be adjusted to the risk of lesional recurrence (e.g., high-risk BCC patients, as is in the case of morpheaform histological subtype; patients already treated for recurrent BCC).
Histological/imaging tests to be performed should be selected according to the suspected disease extent (e.g., local, regional, metastatic), and may include biopsy for histological confirmation of local recurrence, CT scan (if suspected bone disease) or magnetic resonance imaging (MRI) (if perineural invasion, particularly for tumors on the head and neck).
In patients at high risk for multiple primary tumours (e.g., Gorlin syndrome; xeroderma pigmentosum) tighter surveillance is proposed, with skin examination every 4 - 6 months and, given the high risk of extracutaneous abnormalities, additional imaging (e.g., brain MRI, given the high risk of medulloblastoma).
Recent changes on the current standard for the systemic treatment of advanced BCC, emphasize the role of Hh signaling inhibitors as first-line agents, and suggest that immune checkpoint inhibitor-based immunotherapy may be effective in the treatment of this tumor.
In this case, the lack of efficacy of several combined therapeutic strategies turned into a clinical challenge. Advancing the research on the molecular pathology of this kind of unusual tumors will hopefully lead to more succesful outcomes for future patients.