Case Report
A 69-year-old asymptomatic Romanian woman came to our institution for further investigation of a suspected mucinous lesion with gastric fistulization, after performing a routine esophagogastroduodenoscopy (EGD). In that exam, two fistulous tracts in the lesser stomach curvature filled with mucinous content and a bulging of the lesser curvature/posterior gastric wall were visualized. She was further submitted to an endoscopic ultrasonography (EUS), which revealed a predominantly cystic pancreatic lesion with two fistulous tracts to the lesser stomach curvature.
In our institution, a Magnetic Resonance Imaging (MRI) and a Computed Tomography (CT) scan revealed a 13 mm dilation of the main pancreatic duct (MPD) (Figure 1 C, D, E) and adjacent cystic lesions (one represented in Figure 1 A,B) in keeping with mixed-type intraductal papillary mucinous neoplasm (MT-IPMN); two wide communication tracts/fistulas with the stomach were confirmed (Figure 1 A,B,C); enhancing mural nodules were present in the MPD (one represented in Figure 1 E). CT scan also revealed abutment of the mesoportal confluence and hepatic artery and occlusion of the splenic vein. Celiac trunk and superior mesenteric artery were not involved (not shown).
Although she could not recall any symptoms, physical examination showed epigastric tenderness to superficial and deep palpation. Laboratory workup revealed a slight elevation of the CEA (9.3 ng/ml) and CA 19-9 was normal.
As high-risk features were present, surgical treatment was advised. The patient underwent total pancreatectomy with splenectomy, subtotal gastrectomy and resection of the mesoportal confluence with direct vascular anastomosis. The pathological study of the surgical specimen confirmed the diagnosis of malignant transformation of a MT-IPMN with stromal invasion.
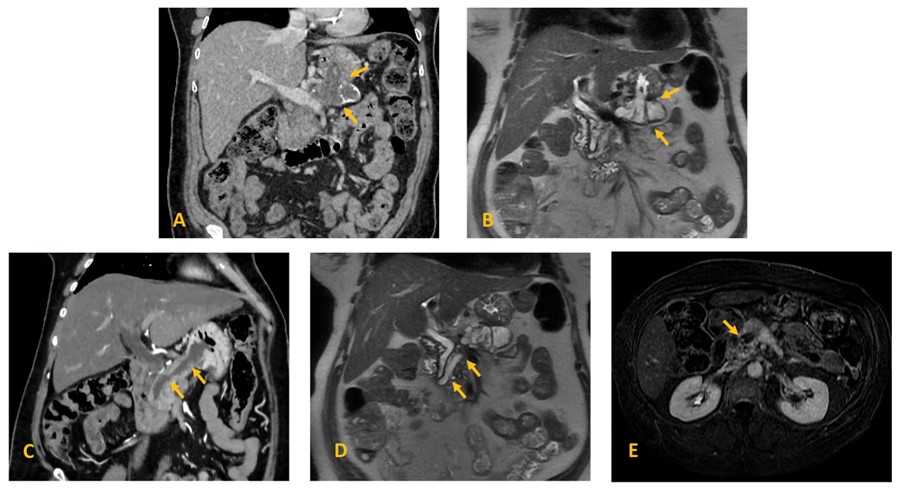
Figure 1: Mixed-type IPMN with two gastropancreatic fistulas in a 69-year-old woman. Coronal (A) contrast-enhanced CT and (B) T2-weighted MR image of a pancreatic cyst with a fistulous tract into the stomach (arrows); (C) contrast-enhanced CT and (D) T2-weighted MR image demonstrate massive dilatation of the main pancreatic duct, with a fistulous tract into the stomach (arrows); (E) Axial contrast-enhanced fat-suppressed T1-weighted MR image on the transitional-phase demonstrate an enhancing nodule within the MD cyst (arrow).
Discussion
IPMNs are pancreatic cystic lesions characterized by a dilated MPD and/or dilated branch ducts with papillary projections composed of dysplastic mucinous epithelium. Dysplastic grades of IPMNs should be identified as low-, intermediate-, or high-grade dysplasia. Histologically, based on mucin expression profiles, IPMNs can be further classified as intestinal, pancreatobiliary, gastric and oncocytic subtypes. Based on the type of ductal involvement, IPMNs can be categorized as branch-duct (BD), main-duct (MD), or mixed-type (MT) IPMNs. BD-IPMNs appear as cysts that communicate with the main pancreatic duct. Main-duct IPMNs, less frequent than the previously discussed, are characterized by segmental or diffuse dilatation of the MPD, ≥ 5 mm, in the absence of other causes of obstruction. Mixed-type IPMN demonstrates characteristics of both main-duct IPMN and branch-duct IPMN.1 MR cholangiopancreatography facilitates the evaluation of IPMNs.1,2
Imaging risk stratification remains an important part of IPMNs clinical assessment. The international consensus Fukuoka guidelines consider high-risk stigmata to be indications for surgical resection, including MPD dilatation ≥10 mm, an enhancing mural nodule ≥ 5 mm, and obstructive jaundice. Patients with worrisome features should undergo endoscopic ultrasound. These include a cyst diameter ≥3 cm, an enhancing mural nodule smaller than 5 mm, a thickened enhancing cyst wall, MPD dilatation of 5-9 mm, an abrupt change in MPD diameter with upstream pancreatic atrophy, lymphadenopathy, and a cyst growth rate of greater than or equal to 5 mm in 2 years.2
Regardless of the malignant transformation of IPMNs, a notable complication associated with IPMNs is the occurrence of fistulization into adjacent organs. Due to limited published data and the often silent progression of this complication, determining its prevalence is challenging. Estimates range from 1.9% to 6.6%, according to two different series.3,4 When symptoms occur, they vary and may include epigastric pain, jaundice and diarrhea.3 This complication often manifests with the presence of multiple fistulous tracts and concurrent involvement of several organs (average of 3.25 fistulas and 1.9 affected organs per patient, in the study of Ravaud et al.). The duodenum is frequently affected, followed by the stomach, bile duct, colon and small intestine.3,4 Cases of fistulization into the spleen and the thigh have also been reported.5,6 The type of IPMN most commonly involved with fistulization is MD-IPMN,3,4 followed by MT and BD-IPMN.3
Based on histological findings, there are two factors presumed to be in the formation of such fistulas: direct invasion of IPMN into other organs due to malignant degeneration and stromal invasion around the fistula; and/or a combination of high pressure in the mucin-filled duct long with inflammatory stimulation. The second factor is not exclusive of benign lesions, as it can occur in malignant cases, when no stromal invasion is documented around the fistula.4
Regarding histological findings, the type of IMPN most frequently associated with fistula formation is the intestinal-type IPMN (MUC2 positive-tumors).3,4 It has been postulated that colloid carcinoma derived from an intestinal-type tumor is deemed to exhibit more expansive and slower progression compared to the other three types of mucin expression, which may explain the high frequency of MUC2 positive-tumors in those with fistula formation.3
In a pre-surgical scenario, accurately determining pancreatic/neighboring structures stromal invasion by a malignant fistulous IPMN might be challenging. Even without histological evidence of stromal invasion at the initial evaluation, it might develop in the long-term.
Nowadays, management and the prognosis of these patients is not yet established, and more research is required.3,4 Kobayashi et al. showed some promising EUS findings that may be useful: the presence of a solid mass in the pancreatic parenchyma and a mass with a mixed-echo pattern in the cystic lumen were confirmed to be highly suggestive of invasive penetrations by malignant IPMN.
In our case, the patient presented with a MT-IPMN with two fistulas to the stomach. Due to the presence of high-risk stigmata, surgical treatment was recommended.
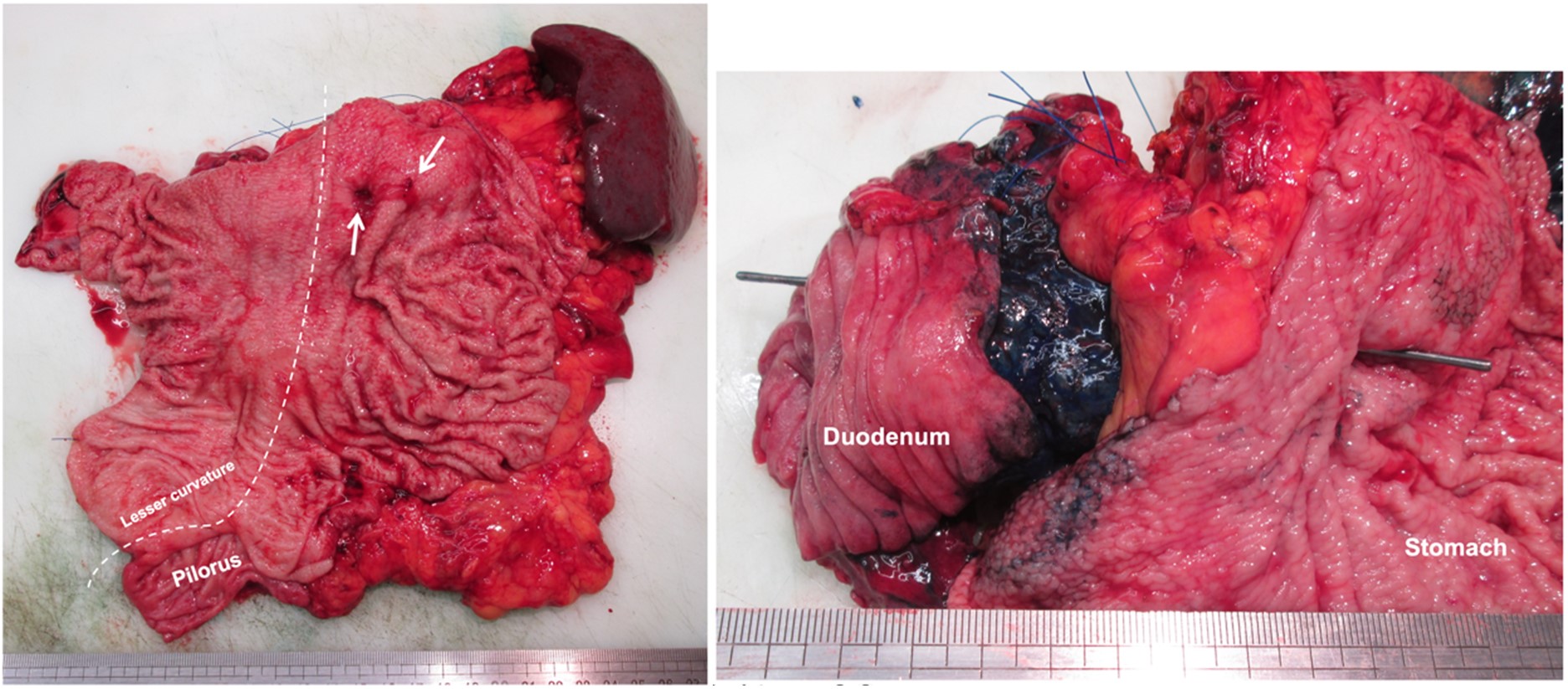
The patient was histologically diagnosed with colloid carcinoma with stromal invasion that had MUC2-positive cells (Figure 2), and therefore, the tumor was deemed to be derived from intestinal type IPMN. The anatomical trajectory of both fistulae were confirmed by macroscopy to come from the MD-IPMN and a BD-IPMN (the first is represented in Figure 3).
Concerning the mechanism of fistula formation, our case showed invasive penetration of the pancreatic stroma and stomach wall.
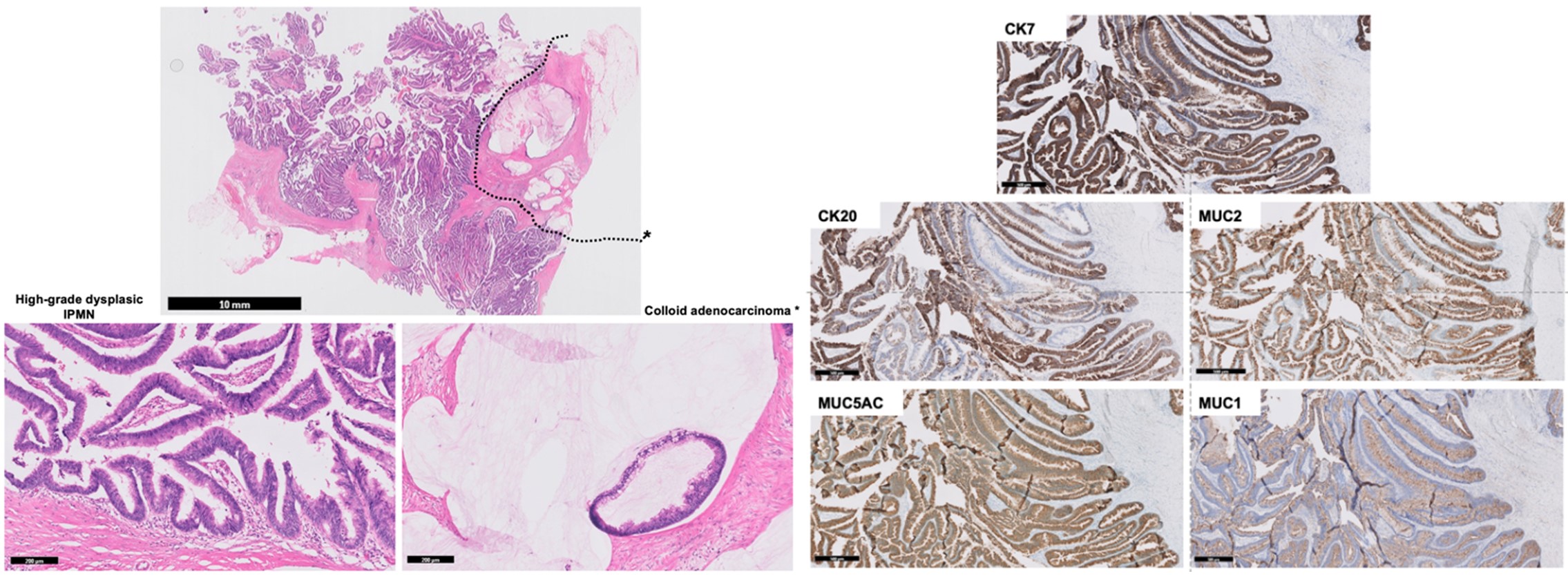
Figure 2: Microphotograph showing an intestinal MT-IPMN with associated colloid adenocarcinoma: (Left, H&E staining) At low magnification (upper image) the lesion corresponds mainly to a papillary neoplasm growing inside the main and secondary ducts with high-grade dysplasia (lower left image), with focal presence of extracellular mucin with floating adenocarcinoma cells corresponding to colloid adenocarcinoma* (delimitated by dotted lines in the upper image and at high magnification in the lower right image); (Right, Immunohistochemical stainings) the intraductal papillary neoplastic cells showed expression of CK7, CK20, MUC2 and MUC5AC, but did not express MUC1, consistent with Intestinal-type IPMN.
Conclusion
A notable complication associated with IPMNs, more commonly MD-IPMN, is the occurrence of fistulous tracts into adjacent organs. In these cases, the high frequency of MUC2 positive-IPMNs (intestinal-type) might be related to a more expansive and slower progression of these lesions.
The long-term consequences of this complication, such as malignant transformation, are not well-established and require ongoing research to determine ideal clinical management.