Introduction
Pancreatic neuroendocrine tumors (panNETs) comprise a heterogenous group of neoplasms with distinct clinicopathological features and long-term prognosis, originating from the islet of Langerhans. PanNETs have been historically regarded as rare, but the reported prevalence in autopsy series (0.8-10%) is much higher than in population-based studies [1, 2]. It is likely that many individuals are affected by small and asymptomatic panNETs that will remain clinically silent for their entire lives [2]. The incidence of these lesions has increased substantially over the recent years, which can be ascribed to the widespread use of abdominal cross-sectional imaging and the increased detection of incidental findings [3]. The earlier diagnosis provided by these diagnostic techniques has been reflected on an increasing overall survival over time [4]. Clinically, panNETs are classified as functional (F-panNETs) or non-functional (NF-panNETs) according to whether they secrete hormones or not. NF-panNETs represent up to 90% of all lesions [5]. Endoscopic ultrasound (EUS) plays a major role in the characterization of these lesions, combining high-resolution morphologic evaluation with the possibility of tissue acquisition. Surgery is the standard of care for otherwise healthy subjects with
larger lesions (>2 cm) or symptoms due to hormone secretion. However, the management of incidentally detected, smaller lesions (<2 cm) remains controversial [6]. EUS-guided therapy, most frequently by radiofrequency ablation (RFA) or ethanol ablation (EA), has emerged as an alternative to surgery in patients with localized disease [7]. This review summarizes the most relevant data regarding interventional therapy for panNETs, namely EUS-guided ablation techniques and surgical resection, pro-viding a clinically oriented insight into the indications and contraindications for each approach, technical specificities, effectiveness, and safety.
Surgical Treatment of PanNETs
The surgical treatment of panNETs has evolved significantly over the last decades. A better comprehension of the biologic behavior and natural history has recently driven to reconsider surgical resection criteria.
Due to the considerable heterogeneity of these lesions, establishing a standard surgical strategy is difficult. It is essential to identify the primary tumor, determine disease extension, and evaluate for respectability.
Tumor grading and staging are the most important independent prognostic factors to be determined before surgery [8]. Figure 1 summarizes the approach to panNETs according to staging and grading of the disease. The benefit from surgery must be balanced against potential post-operative morbidity, mortality, and impact on the functional status after pancreatic resection. Whenever feasible, a minimally invasive surgical approach is recommended, and patients should be referred to experienced surgeons in high-volume centers.
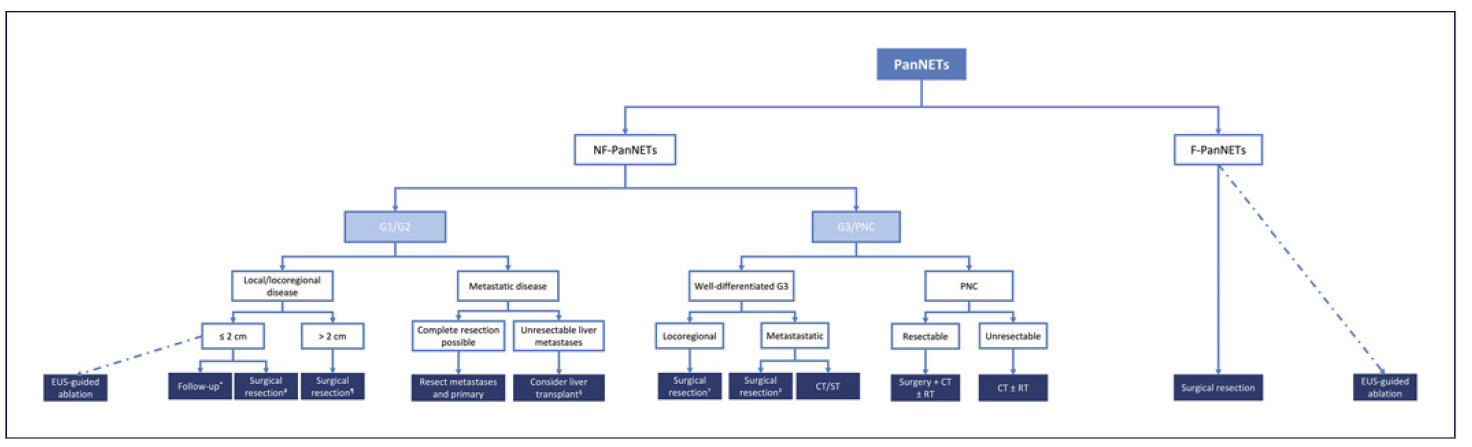
Fig.1 Framework of the treatment of panNETs according to grading and staging. CT, chemotherapy; EUS, endoscopic ultrasound; panNETs, pancreatic neuroendocrine tumors; F-panNETs, functioning pancreatic neuroendocrine tumors; NF-panNETs, non-functioning pancreatic neuroendocrine tumors; PNC, pancreatic neuroendocrine carcinoma; RT, radiotherapy; ST, systemic therapy. *Surveillance may be the preferred option for elderly patients or with significant comorbidities. #Parenchyma-sparing surgery or standard resection ± lymphadenectomy. Consider in younger patients without significant comorbidities or in the presence of signs of local invasiveness, including main pancreatic duct dilation, jaundice, or suspected lymph node involvement. ¶Head: PD + lymphadenectomy; distal lesions: distal resection + splenectomy + lymphadenectomy. §Evaluate: absence of extrahepatic disease, histologic confirmation of a well-differentiated pan-NET (G1/G2, Ki-67 <10%), previous resection of the primary tumor, diffuse liver metastasis involving <50% of total liver volume, stable disease in response to therapy for at least 6monthsand age<60 years old. †Resection + lymphadenectomy. Consider neoadjuvant therapy if unfavorable biology (e.g., Ki-67 >55%). ‡Primary + metastases resection.
Low and Intermediate (G1/G2) PanNETs
Local/Locoregional Disease
Surgical resection is the treatment of choice for local/locoregional disease in panNETs G1/G2. Preoperative evaluation should account for tumor size and location, endocrine functional activity, signs of local invasiveness, and the presence of symptoms [9].
The goal of the surgical treatment of F-panNETs, irrespective of tumor size, should be to provide symptomatic control over the associated clinical syndrome, which can be significant even for small tumors. The symptoms resulting from hormone secretion should be controlled before any intervention [9]. The ultimate goal when resecting F-panNETs is to control the endocrine syndrome and treat the tumor to improve the patients’ overall survival [10].
Curative resection of localized F-panNETs is generally associated with improved long-term survival and a low recurrence risk [11]. Long-term cure rates after R0-resection in localized disease depend on tumor type. For instance, resection of a localized insulinoma results in a 98% biochemical cure rate, with a 6% recurrence rate at 10 years. For sporadic gastrinoma, 60% achieve biochemical response after resection, and 30-40% have disease-free survival at 5 years [12]. For NF-panNETs, surgery should be considered in G1-G3 tumors if symptomatic, larger than 2 cm in size, and/or with atypical features, including irregular margins, heterogeneous echotexture in EUS, and upstream dilatation of the main pancreatic duct [6, 9, 13]. Small NF-panNETs (<1 cm) are often biologically indolent and remain quiescent with time. As such, clinical surveillance is reasonable for patients with asymptomatic NF-panNETs <1 cm [10]. Nevertheless, the decision on whether asymptomatic 1-2 cm NF-panNETs should be resected or kept under surveillance is not consensual, and a case-by-case decision is recommended, acknowledging the patient’s functional status, comorbidities, tumor’s grade, location and growth, risk of developing symptoms, extent of surgical resection, and patient’s preferences [10]. The evolution of EUS-guided local ablative therapies has provided a minimally invasive alternative to surgery for the management of low-grade, small (<2 cm) panNETs.
Currently, for incidentally discovered NF-panNETs <2 cm, surveillance is the preferred option for elderly patients with significant comorbidities and when a pancreaticoduodenectomy is required [9]. On the contrary, surgery should be proposed to younger patients without significant comorbidities or in the presence of signs of local invasiveness, including main pancreatic duct dilation, jaundice, or suspected lymph node involvement [10, 14]. If such signs are present, a standard pancreatectomy with lymphadenectomy is mandatory. A parenchyma-sparing resection can be routinely considered in patients with a long life expectancy [9]. Notwithstanding, considering the potential for morbidity, mortality, and pancreatic insufficiency after resection and the low risk of malignancy, surveillance might be a reasonable strategy for NF-panNETs <2cmwith a low proliferation index, defined as Ki-67 index <5% in EUS-guided biopsy samples (preferably acquired using an end-cutting type FNB needle) [14, 15].
For NF-panNETs >2 cm, surgical resection is recommended [10]. Either standard resection or parenchyma-sparing surgery might be considered appropriate, with or without regional lymph node removal, based on preoperative staging. NF-panNETs >2 cm are at increased risk of nodal metastasis, and some authors recommend that a standard pancreatectomy with regional lymphadenectomy be performed [9].
Advanced/Metastatic Disease
The major cause of death in patients with neuroendocrine tumors is liver failure from diffuse hepatic metastases, which is particularly frequent for panNETs. Extrahepatic disease rarely leads to a patient’s death, so its presence should not contraindicate primary tumor resection and/or hepatic cytoreductive surgery [10]. Patients with metastatic disease may still have a favorable survival outcome after cytoreductive surgery [10]. The decision to resect a primary panNET in the setting of metastatic disease should be made on an individual basis, accounting for age and comorbidities, tumor functional status, location, intention to treat or potential local complications from the tumor, and the possibility to improve the response to medical therapy [10]. In fact, F-panNETs might derive more benefit, and lesions located in the tail and body of the pancreas are more favorable to resection than those in the head due to lower surgical morbidity from distal pancreatectomy compared with pancreatoduodenectomy (PD). Despite not being consensual, most experts are in favor that primary tumor resection may be beneficial in selected cases of metastatic disease [10]. Recent studies report that in panNETs, lymph node status and the number of nodes resected may have prognostic value, with a potential impact on reducing persistent disease and improving survival [6]. In the case of metastatic disease, patients with F-panNETs with a high tumor burden may benefit from cytoreductive surgery. On the other hand, the need for palliative resection in NF-panNETs is debatable, as the risk of tumor-related symptoms is low (and it is not considered in tumors with Ki-67 >10%). Nevertheless, recent evidence from retrospective series suggests that primary tumor resection can be associated with better long-term outcomes [16]. As such, for tumors with proven lymph node metastases, surgery can be recommended. In cases of G1/G2 panNETs with liver metastasis, a favorable response with survival benefit might derive from hepatic cytoreductive surgery. Timing for recurrence or tumor progression after hepatic cytoreductive surgery has been reported to vary between 11 months and more than 3 years, depending on tumor burden, surveillance schedule, and imaging modality [10]. However, the timing of surgical cytoreduction remains debatable. Currently, no data exist favoring an observation period prior to cytoreduction surgery to allow for new metastases to develop, and both surveillance and immediate surgery (as soon as metastases become evident) are acceptable options [10].
Poorly Differentiated High-Grade Pancreatic Neuroendocrine Carcinoma
In localized poorly differentiated (always considered high-grade - G3, Ki-67 >20%) pancreatic neuroendocrine carcinoma, the role of surgical resection remains controversial as it may not have clear survival benefits as an initial approach [17]. The presence of high-risk features (large tumor size and/or high-grade poorly differentiated tumor) should discourage an upfront surgical approach. For these patients, despite the lack of evidence, neo-adjuvant treatment may be considered. Nevertheless, in selected cases of resectable disease, a radical surgery followed by adjuvant chemotherapy (CT) with or without radiotherapy can be performed. For locoregional non-resectable disease, patients should undergo neoadjuvant locoregional chemoradiation therapy. A combination of platinum-based CT with local treatment consisting of radiotherapy and/or surgery probably offers the greatest likelihood of long-term survival [10]. For patients with distant metastases or advanced non-resectable disease, CT is the preferred treatment option, provided the patient has adequate organ function and performance status [18]. Radiotherapy might be considered for lesions in selected locations, such as for brain and bone metastases, to provide symptom control. Debulking or surgical resection of metastasis is not recommended.
Well-differentiated G3 panNETs, if localized, should be evaluated for resection, in the setting of a multimodal therapeutic strategy [10]. Curative surgery is usually attempted in these cases, although retrospective series indicate that it is rarely curative as a single modality [19]. In patients with hepatic metastases, cytoreductive surgery may not be advisable due to high recurrence rates and poor survival. Thus, in this setting, CT should be considered a first line. Further studies are needed to clarify whether patients with G3 panNETs and lower Ki-67 (21-55%) may benefit from a more aggressive surgical approach [10]. Some authors propose neoadjuvant CT followed by definitive surgery, although data are scarce [20]. Most patients with pancreatic neuroendocrine carcinoma have significant extrahepatic disease or non-resectable liver metastasis (or not suitable for complete resection), thus having no benefit from undergoing surgery, owing their poor survival [10, 13].
Syndromic PanNETs
Hereditary syndromes often occur with multiple panNETs, which poses complex challenges. The goal should be the removal of the dominant lesion and, potentially, other easily accessible lesions. This should be counterbalanced with the need to preserve as much pancreatic tissue and function as possible. It should be considered to intervene prior to malignant progression while minimizing surgery-related morbidity and mortality [21].
Individual factors, such as comorbidities and the potential need for multiple surgeries to treat multifocal or metachronous tumors, should be considered when choosing optimal surgical timing and extension. Broad principles for the management include the performance of parenchyma-sparing procedures, watchful surveillance for low-risk tumors, enucleation or minimal pancreatic resection for intermediate-risk tumors (when feasible and effective), and major resections reserved for locally invasive, anatomically difficult, or high-risk lesions [10].
Which Surgery Should Be Performed for PanNETs?
Oncological outcomes for panNETs patients are widely heterogeneous depending on factors such as tu-mor grading, size, and staging. A recent meta-analysis reported a 5-year disease-free survival >90% in resected patients without synchronous liver metastasis [11]. If liver metastases are present and a R0-resection is performed, overall 5-year survival can be up to 85% [11]. However, despite excellent overall survival, tumor recurrences after surgery are frequent. After a R0-resection, cumulative recurrence incidence reaches 27% at 3 years and 40% at 5 years [22]. When surgery is considered, two strategies can be discussed: standard resection, with standard lymphadenectomy, and parenchyma-sparing surgery, possibly with lymph node sampling.
Standard Resection
Standard resection, distal pancreatectomy, or PD should be performed for panNETs at risk of nodal involvement or if signs of local invasion are present. It is recommended in cases of NF-panNETs >2 cm and F-panNETs other than insulinoma [13]. Regional lymphadenectomy is mandatory since lymph node involvement has significant prognostic implications. Removal of 11-15 lymph nodes should be performed for accurate nodal staging [23]. For left-sided tumors, depending on their anatomical relation with the splenic hilum and vessels, spleen-preserving distal pancreatectomy can be considered for small, presumably benign panNETs [24]. However, for large tumors or tumors invading the splenic vein and/or surrounding structures, splenic preservation is hardly possible [25]. Regarding short-term outcomes, minimally invasive distal pancreatectomy is the preferred approach to tumors confined to the distal pancreas, with no local invasion and <8cm in size [25]. A recent meta-analysis estimated the median prevalence of exocrine pancreatic insufficiency at 22% after standard resection, most commonly after PD (median prevalence of 43%) [26]. Endocrine insufficiency, despite being less common, still affects 20% of patients submitted to standard pancreatectomy [27].
Parenchyma-Sparing Surgery
Parenchyma-sparing surgery, such as enucleation and central pancreatectomy, is seen as an alternative for small, low-grade tumors and might be an option for NF-panNETs <2 cm and insulinomas [13, 28]. Despite significant postoperative morbidity, these procedures achieve a recurrence-free 5-year survival of >95% in selected panNETs [13]. Minimally invasive approaches are technically feasible, safe, and may have potential advantages over open resection in experienced centers [10]. When limited resection is considered, the removal of suspicious nodes identified on preoperative imaging is warranted, and lymph node sampling may be considered to assess for nodal invasion if imaging is negative.
Enucleation is associated with improved pancreatic function but at a cost of a higher rate of postoperative pancreatic fistulae. Criteria for patient selection have not been defined, but expert’s opinions suggest that enucleation should be reserved for smaller tumors, those more likely to display benign behavior, and those located >2-3mmfrom the main pancreatic duct [10, 13]. For instance, F-panNETs ≤2 cm represent the most appropriate lesions to be enucleated, given their safe distance from the main pancreatic duct [9].
For central pancreatectomy, the primary indication is deeply located, small, benign, or low-grade panNETs in the neck or proximal body of the pancreas that are not suitable for enucleation due to their proximity to the main duct. It is necessary to assure a pancreatic remnant long enough to maintain normal pancreatic function. A 5-cm-length segment is usually required [10]. Despite more favorable in preserving pancreatic function opposed to standard resections, this has to be balanced with a higher overall morbidity and pancreatic fistulae rate [10, 13]. Patients with larger lesions, diffuse pancreatitis, and high-grade tumors are not candidates for central pancreatectomy [10, 28].
Usually, <5% of patients develop pancreatic insufficiency [16]. These better functional outcomes in relation to standard resections have to be balanced against an increased rate of postoperative morbidity, especially pancreatic fistulae, which, in some prospective, studies reaches 45% [13, 16]. Thus, this procedure may be more appropriate for younger surgical-fit patients.
Resection Margins
There are neither randomized trials nor large series examining the impact of resection margins on local recurrence [10]. Tumor biology rather than margin status appears to impact survival [29].
Negative surgical margins should be the goal, although no conclusive data support the benefit of a more aggressive strategy. In fact, more aggressive strategies might be associated with higher morbidity, and, in selected patients, parenchyma-sparing procedures with minimal margins are reasonable options to prevent morbidity and maintain normal pancreatic function [10].
Hepatic Cytoreductive Surgery
There is no consensus regarding the role of cytoreductive hepatic surgery in patients with liver metastasis [10]. Recent studies have challenged the idea that >90% of liver metastases must be resected in order to either palliate or improve survival of patients with neuroendocrine liver metastases. It is easier to achieve higher levels of cytoreduction in patients with fewer liver metastases, but good results have been obtained even in patients with >10 lesions, and published data have shown potential survival benefits [10].
Combined Pancreatectomy and Hepatic Cytoreductive Surgery
Some patients with panNETs and liver metastases may be eligible for both primary tumor resection and hepatic cytoreductive surgery. Combining these procedures de-pends on the extent of planned pancreatic and liver re-section and is a reasonable approach if the patient’s comorbidities and functional status do not contraindicate it [8, 10]. Indeed, the morbidity of a PD ranges up to 37%and that of a hepatic resection up to 12% [30]. Nevertheless, several reports suggest that synchronous resections can be performed safely, with acceptable morbidity and mortality in selected patients, when performed by experienced surgeons. Alternatively, combining liver resection and RFA may provide the opportunity to achieve complete tumor ablation with more limited resections, thus minimizing the impact on the residual liver function [9].
Liver Transplantation
Liver transplantation may be an option in very selected patients with unresectable panNET liver metastasis [31]. Some criteria to be considered are the absence of extrahepatic disease, histologic confirmation of a well-differentiated panNET (G1/G2, Ki-67 <10%), previous resection of the primary tumor, diffuse liver metastasis involving <50% of total liver volume, stable disease in response to therapy for at least 6 months, and age <60 years old [32]. In patients that fulfilling these criteria, a 5-year overall survival of 69-97% after transplantation has been reported [32].
EUS-Guided Ablation of PanNETs
EUS allows the accurate visualization of the pancreatic parenchyma and has become a key modality for the characterization of pancreatic neoplasms, including panNETs. EUS allows for complementary diagnostic procedures, including tissue acquisition as well as lesion localization through EUS-guided fiducial placement or tattooing using sterile carbon-based ink [33, 34].
Over the last years, EUS has evolved from a purely diagnostic procedure toward an interventional modality. With the development of dedicated endoscopic devices, a lot of interest has been devoted to the possibility of delivering loco-regional treatment, avoiding the need for surgical treatment. In the case of panNETs, the treatment goals differ significantly according to their classification as F-panNETs or NF-panNETs. Indeed, as F-panNETs have a low risk of malignant transformation (particularly insulinomas), the main goal when treating these lesions is to provide adequate control of the endocrine disturbance. On the other hand, local ablative therapies for NF-panNETs should attain complete tumor ablation [35]. Two techniques have been most extensively evaluated for EUS-guided ablation of panNETs: EUS-guided RFA (EUS-RFA) and EUS-guided EA (EUS-EA).
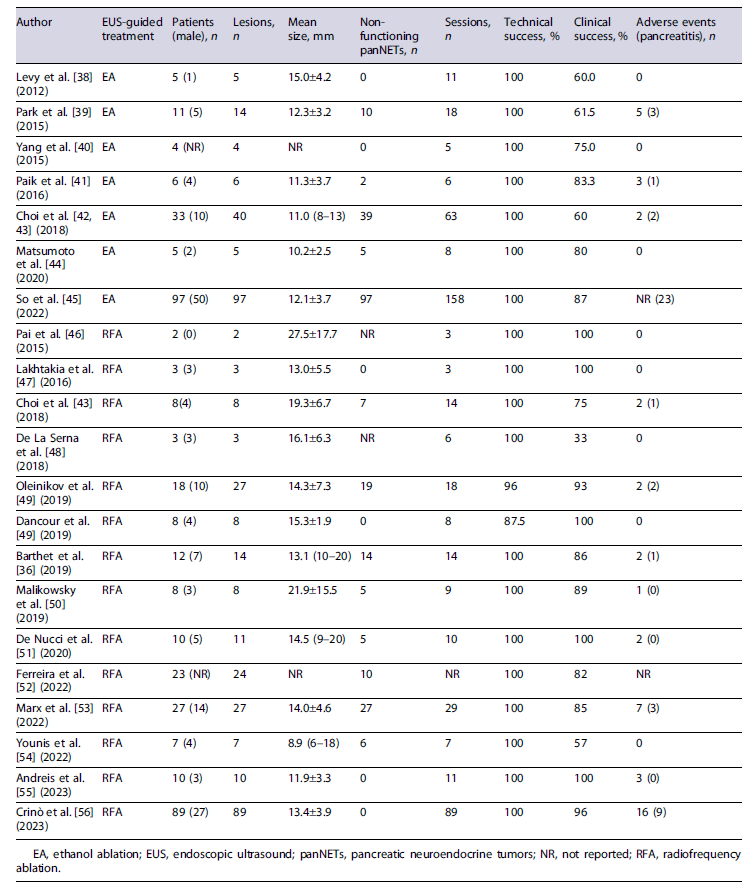
Patient selection is critical for the safe and effective use of this procedure. Experience with these techniques remains limited to small series, and no large prospective studies or randomized clinical trials exist on this subject [7]. Currently, the European Neuroendocrine Tumor Society (ENETS) guidelines include EUS-guided ablative therapies as an alternative to surgery in selected patients, although its routine use is not endorsed [6]. Therefore, the use of these techniques should still be considered investigational. Currently, patients with lesions up to 2 cm in size, low grade (G1) in the World Health Organization classification, as well as patients who are unfit or unwilling to undergo surgery, are included in trials on EUS-guided ablative therapy (Fig. 1) [36, 37]. Additionally, comorbidities and overall life expectancy should be considered before offering ablative therapy for any given patient. Clinical success definitions differ according to the secretory phenotype of the panNET. For F-panNETs, most studies define clinical success after ablative therapy as the complete symptomatic resolution during follow-up. The definition of clinical success after EUS-guided ablation of NF-panNETs is more variable, although most studies agree on the complete disappearance of the lesion, as determined by the absence of arterial phase contrast enhancement in computed tomography or magnetic resonance imaging [7]. Table 1 provides a summary of the studies evaluating the feasibility, performance, and safety of EUS-guided ablation of panNETs.
EUS-Guided Radiofrequency Ablation
EUS-RFA consists of the application of a high-frequency alternating current to generate thermal energy to provide local tissue destruction [57, 58]. This technique takes advantage of the thermosensitivity of the pancreatic tissue. The friction generated by the alternating current produces heat, inducing protein denaturation followed by intracellular dehydration and coagulative necrosis [59]. Additionally, there is some evidence of indirect thermal injury, including loss of membrane integrity, mitochondrial dysfunction, oxidative stress, and a delayed immune response [60, 61]. The RFA probe induces active heating of the few millimeters around the electrode and, when applied in excess (temperatures >100°C), tissue dehydration and charring occur around the probe, which act as insulators, leading to a rise in impedance and decreasing the efficiency of the procedure [62, 63].
Tissues farther from the electrode are heated by passive thermal conduction, which may not be sufficient to induce the rise in temperature required to cause necrosis [59]. Thus, one significant concern regarding this technique is the limited extent of necrosis, which may not be enough to cover all the tumor volume [59]. RFA can be applied through monopolar or bipolar probes, the latter being able to produce more rapid and focal heating, mitigating injury to adjacent tissues [62].
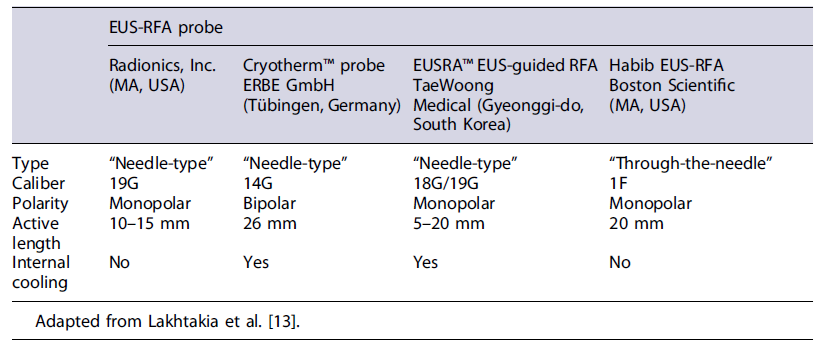
Ablation of panNETs by RFA is performed using conventional linear echoendoscopes. Several probes have been developed (Table 2), both coupled to a needle (needle-type) or for through-the-needle placement, in which the probe is passed into the tumor through a 19G needle. The EUSRA-RFA probe (Taewoong Medical Co., Gyeonggido, South Korea) has an internal cooling system to prevent tissue charring [57]. Real-time EUS control of the procedure is paramount, and doppler should be applied to mitigate the risk of vascular damage. Care should be taken to avoid extensive damage to normal parenchyma and to the wall of the gastrointestinal tract. It is recommended to start RFA at the farthest and deepest border of the lesion, since EUS-RFA induces image artifacts that can hamper the completion of the procedure. For large lesions, a fanning technique could be applied to treat the entire tumor [59]. Treatment should be withheld when hyperechoic bubbles extend beyond the lesion or when impedance increases [35].
EUS-RFA protocols vary significantly between centers (including power settings and duration of the ablation), and the existing evidence is based on studies that include a limited number of patients [7]. One of the largest prospective cohorts included 12 patients, with a total of 14 NF-panNETs [36]. This group reported a rate of significant response (decrease in diameter >50% or tumor disappearance) at 1-year follow-up of 86% [36]. These results were also observed after a longer follow-up period, as recently reported [37]. Technical success was achieved for all lesions [36]. A recent meta-analysis including 13 EUS-RFA studies (n = 113 patients) reported pooled rates of clinical and technical success of 85% and 94%, respectively [7]. Lesion size ≤18 mm and location in the head of the pancreas appear to predict higher ablation success rates [7, 64]. A recent case series including 10 patients with pancreatic insulinoma suggests that EUS-RFA presents high levels of clinical success for the treatment of
F-panNETs. In this series, technical success was achieved in all patients, with 90% requiring one EUS-RFA session. All patients remained free of hypoglycemia events [55].
Globally, EUS-guided RFA of panNETs appears to be a relatively safe procedure. Only 12 adverse events were reported in the previously cited meta-analysis (pooled rate of 14%), most commonly acute pancreatitis (pooled rate of 8%) and abdominal pain [7].
EUS-Guided Ethanol Ablation
Chemoablation with ethanol has been widely used for the treatment of a wide range of cystic and solid lesions, including renal cysts and hepatocarcinoma [62, 65]. EUS-EA is inexpensive and induces coagulation necrosis, cell lysis, protein denaturation, and vascular thrombosis, subsequently leading to fibrosis [66, 67].
Regarding the technical aspects of this technique, the ablation agent is delivered by a small gauge EUS-FNA needle (22G or 25G), and small volumes of ethanol are injected within the tumor, which can be repeated until a hyperechoic blush is observed. The first-ever application of EUS-EA for a solid pancreatic lesion was reported in 2006 for the treatment of a symptomatic insulinoma [68]. A 95% ethanol solution was used, with complete symptom disappearance and endosonographic resolution of the lesion being achieved.
In general terms, this technique appears to be effective and safe. Indeed, one of the largest cohorts including 33 patients with a total of 40 tumors, reported a complete ablation rate of 60% after injection with 99% ethanol, with no recurrence during follow-up and an adverse event rate of 3.2% [42]. Nevertheless, the technique is not standardized, and there is currently no recommended protocol for the application of this ablative therapy. This is particularly evident for ethanol concentration, which varies largely between 40% and 99% in different reports. A recent systematic review with meta-analysis of 7 studies on EUS-EA, including a total of 91 patients, reported a pooled rate of technical and clinical success of 97% and 82%, respectively [7].
One of the main concerns regarding this technique is the injection of ethanol intra- or retroperitoneally or at the adjacent normal pancreatic parenchyma. The rate of adverse events after EUS-EA is reported to be around 12%, with a pooled rate of pancreatitis of 8% [7].
The efficiency and long-term effects of EUS-EA are still not completely understood as current evidence is based on case reports and small case series. At this point, this technique is not included in formal treatment and follow-up guidelines on panNETs but should be considered for patients requiring treatment and who are unfit for surgery. Future studies should provide evidence regarding the influence of different ethanol concentrations, needle sizes, and follow-up protocol.