Introduction
Desmoid tumor (DT) is a rare mesenchymal neoplasm of unknown etiology. Most cases are sporadic (85-90%) and related to somatic mutations in CTNNB1 gene, while 5-10% arises from a germline mutation in APC gene, in the context of familial adenomatous polyposis (FAP) [1]. Previous trauma, abdominal surgery, pregnancy, and hormonal therapy are known risk factors for DT development in sporadic cases [2]. Typically, DT is characterized by slow growth and no potential of metastasis but is known to be locally aggressive, leading to significant morbidity and mortality. Despite margin-free surgical resection, DTs have a high recurrence rate, ranging between 5 and 67% [3]. A recent meta-analysis reported a recurrence rate of 17.7% [4]. Over the last years, an increased number of DT development after bariatric surgery have been reported [5-9]. The increasing number of bariatric surgery procedures performed worldwide, owing to the alarming growing incidence of obesity and related conditions [10], may explain the raise in DT diagnosis related to bariatric procedures. Herein, we report a case of an intra-abdominal DT developed 3 years after sleeve gastrectomy.
Case Report
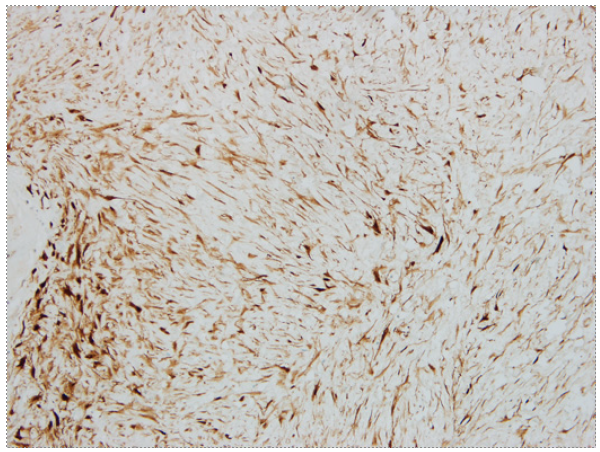
A 26-year-old male with a past medical history of vertical sleeve gastrectomy 3 years before, presented to the Gastroenterology consultation with abdominal pain and postprandial fullness of progressive worsening during the last 2 months. He denied associated symptoms such as nausea, vomits, fever, or weight loss. He also denied other medical conditions or a family history of FAP. Abdominal ultrasound revealed a bulky intra-abdominal mass that prompted further investigation. Abdominal computed tomography confirmed the presence of an intra-abdominal homogeneous mass (40 × 21 × 11.7 cm), centered in the mesentery, causing compression of the adjacent organs but without radiological signs of local invasion. No signs of bowel distension/obstruction or suspicious adenopathy were observed (Fig. 1). Percutaneous biopsy with an 18-gauge tru-cut needle was per-formed. Histopathological examination revealed fusiform cells arranged in bundles with beta-catenin expression (negative for desmin, DOG-1, CD117, and CD34), suggestive of a mesenquimatous tumor. Considering the tumor dimension and the symptomatic course of the disease, after discussion of the treatment options with the patient, elective surgery was decided. Laparotomy revealed a large mass arising from the mesentery root in intimal contact with a jejunal loop. After ligation of the vessels responsible for the tumor supply, segmental ischemia of the adjacent jejunal loop occurred. Thus, a 10 cm segmental enterectomy was also performed. The postoperative course was uneventful, and the patient was dis-charged 5 days later. The resected specimen measured 40 × 22.5 × 14.5 cm and weighed 5,550 g. Macroscopic evaluation revealed a smooth and brownish-white capsule with a homogeneous and whitish gelatinous core, with focal hemorrhagic areas. Histopathological examination showed fusiform to stellate cells with mild atypia and thin-walled vessels (Fig. 2). Immunohistochemical staining demonstrated diffuse beta-catenin expression, in the absence of expression for DOG-1, CD117, CD34, S100, desmin, and alpha-actin, consistent with desmoid-type fibromatosis (Fig. 3). Invasion of adipose tissue and small intestine wall (muscularis propria and submucosa) was present. Resection margins were negative. After 4-year follow-up, the patient remains asymptomatic without evidence of local recurrence.
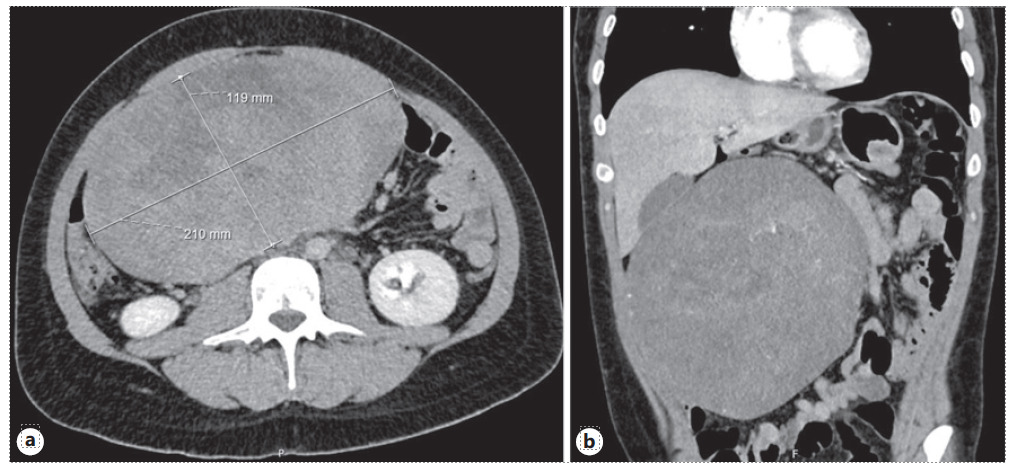
Fig. 1 a Bulky intra-abdominal mass, measuring 21.0 × 11.9 cm in axial axis, centered in mesentery. b Causing compression in the small bowel without imaging features suggestive of intestinal occlusion.
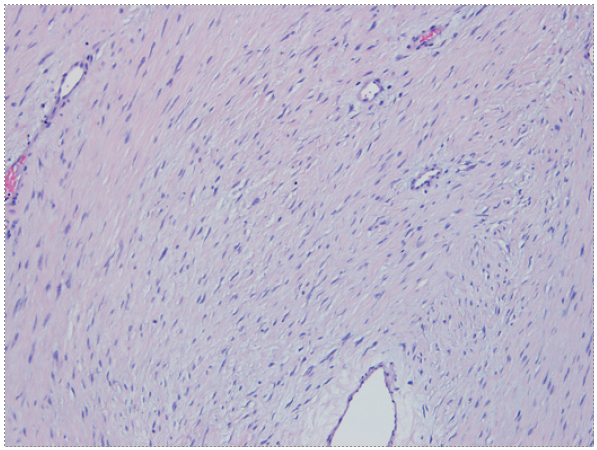
Fig. 2 Fusiform to stellate cells with mild atypia and thin-walled vessels. Presence of myxoid stroma, without mitosis or necrosis (×200).
Discussion
DTs are a rare neoplasm derived from mesenchymal origin, with an estimated incidence of 2-4per million, accounting for 0.03% of all neoplasms [11]. The peak of incidence occurs between 30 and 40 years and is more common among women [12]. DTs etiology is not fully understood, but Wnt pathway plays a key role in DT pathogenesis [13]. Both sporadic DT and FAP-associated DT are linked to constitutive activation of the Wnt signaling pathway, resulting in cytoplasmic accumulation of β-catenin, followed by its translocation into the nucleus. Ultimately this results in the overexpression of genes involved in proliferation, fibrosis, and angiogenesis [14]. Since Wnt pathway has an active role in wounding repair [15], emerging data suggest that there is a dysregulated healing process after trauma (surgical or not) that favors DT development [14]. DTs can occur anywhere in the body but are more frequent in the abdominal wall, intra-abdominal cavity, and limbs [12]. Clinical presentation is variable, depending on its location, size, and growth rate. Intra-abdominal tumors are initially asymptomatic until they reach large dimensions, causing abdominal pain, palpable abdominal mass, bowel obstruction, ischemia, and rarely perforation or bleeding [12]. Focusing on DTs after bariatric surgery, Table 1 summarizes the described cases available in the literature. Abdominal pain is the most frequent symptom (100%), followed by palpable abdominal mass (40%)[5-9]. All previously reported cases revealed an abdominal mass of at least 10 cm, confirming the potential of local aggressiveness. These tumors mostly occurred in women (80%) and the median age at diagnosis was 44 years (interquartile range 10). Median time from surgery to diagnosis was 2 years (interquartile range 0.5) [5-9].
Imaging studies are usually the initial diagnostic method, followed by histopathological characterization of tissue sample to confirm the diagnosis [16]. When feasible, surgical resection used to be the first-line approach. More recently, The Desmoid Tumor Working Group suggest active surveillance for asymptomatic patients regardless of tumor size [1]. Surgery may be considered ad initium in symptomatic patients or critical locations such as mesentery or head and neck[1].Duringactive surveillance, an active treatment, either surgery or medical therapy, should only be considered in case of persistent progression (especially if become symptomatic or is located in critical sites) [1]. However, the choice of treatment should be individualized according to patient and lesion characteristics. In some cases, radiotherapy or chemotherapy alone or combined with surgery may also be an option [1].
All previously reported cases were approached surgically, without significant morbidity or mortality [5-9]. Negative margins on surgical specimens (R0 resection) were reported in 3 cases [5, 7, 8]. Absence of recurrence was reported in only 1 case, but follow-up time was not reported [8]. In our case, R0 resection was achieved, and the patient did not develop complications related to surgery despite a locally advanced tumor. No recurrence was seen 4 years after DT removal.
To our knowledge, this is the first literature review approaching the development of DTs after bariatric surgery. With the increasing number of bariatric procedures performed worldwide, it is expected that DTs reports will gradually increase over the next few years. Thus, both gastroenterologists and surgeons should be aware of the potential for DT development shortly after surgery, to offer a prompt diagnosis and treatment.