Introduction
Colorectal cancer (CRC) is the third most frequent cancer and it is responsible for 10% of cancer mortality worldwide [1, 2]. This cancer type occurs mostly from conventional adenoma (CA)-carcinoma pathway, while serrated pathway is responsible for about 25% of the cases [3]. Serrated lesions (SLs) are known as the precursor lesion in this carcinogenic pathway. SL can be divided into hyperplastic polyps (HPs), sessile serrated lesions (SSLs), sessile serrated lesions with dysplasia (SSLs-D), traditional serrated adenomas, and unclassified serrated adenomas, accordingly to the World Health Organization [4].
The last two decades were marked by advances in the study of the serrated pathway in order to understand the neoplastic mechanisms underlying this disease to prevent the progression to cancer [5]. This pathway is characterized by epigenetic alterations with mismatch repair genes deficiency and by the presence of a CpG island hypermethylation phenotype, with microsatellite instability in the vast majority of the cases [1, 6, 7]. Consequently, the serrated pathway presents a high lymphocytic immune infiltrate and upregulation of immune checkpoints associated with tumor immune evasion [8]. However, there is a gap of knowledge in understanding this pathway, particularly the progression to malignancy and the risk factors involved.
Some association studies defined smoking, alcohol consumption, overweight, red meat consumption, hypertension, and hypertriglyceridemia as risk factors for the SL development. Other researchers identified aging, absence of regular consumption of non-steroidal anti-inflammatory drugs, polyp dimensions ≥10 mm, dysplasia, female gender, and synchronous CA as risk factors for progression to malignancy in the serrated pathway [7, 9-15]. However, these risk factors are not as well established as in the adenoma-carcinoma pathway. Additionally, this CRC subtype is one of the responsibilities for the occurrence of interval cancers. This is presumed to be secondary to the difficult endoscopic identification of these lesions due to their sessile morphology, mucus coverage, and proximal colon location and rapid cancer progression after the development of dysplasia [7, 9, 10].
Thus, the protective effect of CRC screening is expected to decrease in these patients [7, 10]. This represents a challenge to physicians managing these cases due to the lack of biomarkers that could impact therapeutic decisions. Taking this into account, it seems crucial to identify a new biomarker capable of improving risk stratification and further clinical decision.
N-glycosylation has been associated with the malignant transformation process, and it is considered to be a cancer hallmark [11]. This process is a post-translational modification characterized by enzymatic reactions that allow the binding of carbohydrates (glycans) to proteins, lipids, or other saccharides [11]. These glycan structures are found on all cell surfaces, constituting the glycocalyx [12]. The differential glycans profiles are associated with immunologic and epithelial biologic functions [13]. In fact, our group described that the expression of β1,6-GlcNAc branched N-glycans in the conventional colorectal carcinogenesis cascade is considered an important immune checkpoint, demonstrating that these complex N-glycans overexpression in CRC cells was associated with immune escape [14]. Additionally, Demetriou et al. [15] demonstrated that T-cell activity is particularly regulated by β1,6-GlcNAc branched N-glycans on the T-cell receptor that modulates the threshold of T-cell activation and signaling. In line with this and in the context of chronic inflammatory processes such as inflammatory bowel disease, our group showed that these complex N-glycans are capable of regulating T-cell-mediated immune response associated with disease severity [16]. Particularly, we demonstrated that a β1,6-GlcNAc branched N-glycans deficiency, due to a MGAT5 decreased expression, confers an hyperimmune response by decreasing T-cell activation threshold, increasing proinflammatory cytokines production, and increasing T-cell signaling [16]. This highlights the crucial role of the N-glycans patternoncancer development/progression and immune response regulation. Therefore, in this study, we aimed to identify risk factors for progression to malignancy of serrated pathway lesions (SPLs) based on the N-glycosylation profile of both cancer cells and infiltrating immune cells.
Methods
Cohort Characterization
This is retrospective cohort study of patients with lesions of the serrated pathway, followed between September 2014 and 2021. Data were collected from 53 colonoscopies, corresponding to 48 patients.
The N-glycosylation profile was previously obtained from FFPE (formalin-fixed paraffin-embedded) biopsies and fresh biopsies of SPL. The MGAT5 gene (gene that encodes the enzyme N-acetylglucosa-minyltransferase-V [GnT-V]), responsible for the expression of β1,6-GlcNAc branched N-glycans, was evaluated by reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) in FFPE biopsies. Also, in FFPE biopsies, a lectin histochemistry was performed to evaluate the expression of β1,6-GlcNAc branched N-glycans (complex glycans) in epithelial and stromal cells, obtained by staining with Phaseolus vulgaris leucoagglutinin (L-PHA), as well as the presence of high-mannose N-glycans (simple glycans), identified by labeling Glanthus nivalis agglutinin. The lectin histochemistry evaluation was performed by two independent observers, who gave a score from 0 to 3 according to the degree of staining (0: ≤25%; 1: 26% to 50%, 2: 51% to 75%, and 3: >75%). Flow cytometry of epithelial cells (CD45− cells), CD4+ T cells, CD8+ T cells, and FoxP3+CD25+ T cells (regulatory Tcell- Treg) was performed on fresh biopsies, and L-PHA and Glanthus nivalisagglutinin expression was obtained, corresponding to β1,6-GlcNAc branched N-glycans and high-mannose N-glycans ex-pression, respectively, in each of these cell populations.
Statistical Analysis
Descriptive statistics were performed based on the analysis of the mean and standard deviation of the continuous variables under study; percentages were used for the categorical variables. The relationship between the clinical variables and the N-glycosylation profile of the epithelial cells and colonic T cells obtained by RT-qPCR, lectin histochemistry, and flow cytometry was performed using Pearson’s correlation, t test for independent samples, and nonparametric Mann-Whitney U. This relationship was performed for 3 groups: SPL (which includes SL and serrated pathway adenocarcinoma), SL (which includes HP, SSL, and SSL-D), and SSL (with or without dysplasia). The association between continuous variables and non-binary discrete variables was performed using the one-way ANOVA test, using Tukey’s post hoc test. A significance level of 0.05 was considered and the statistical analysis of the variables was performed using the SPSS version 26 program.
Results
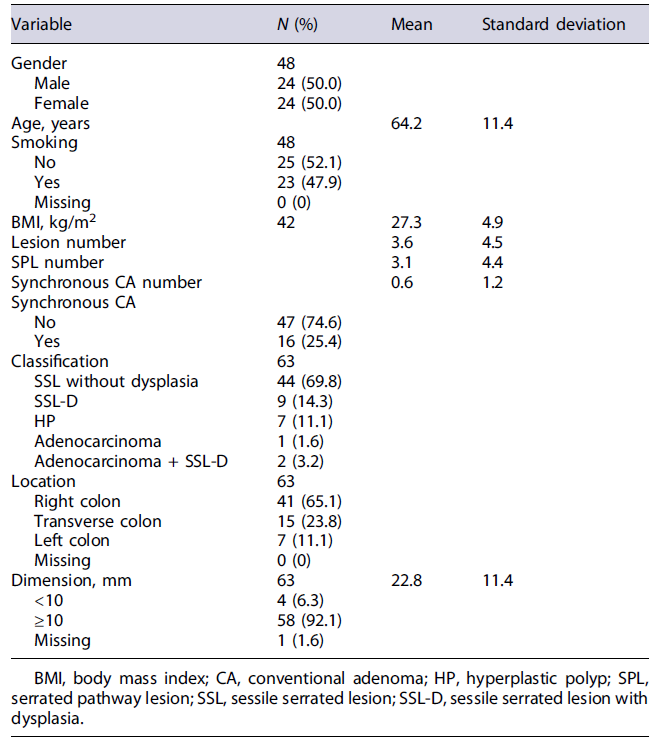
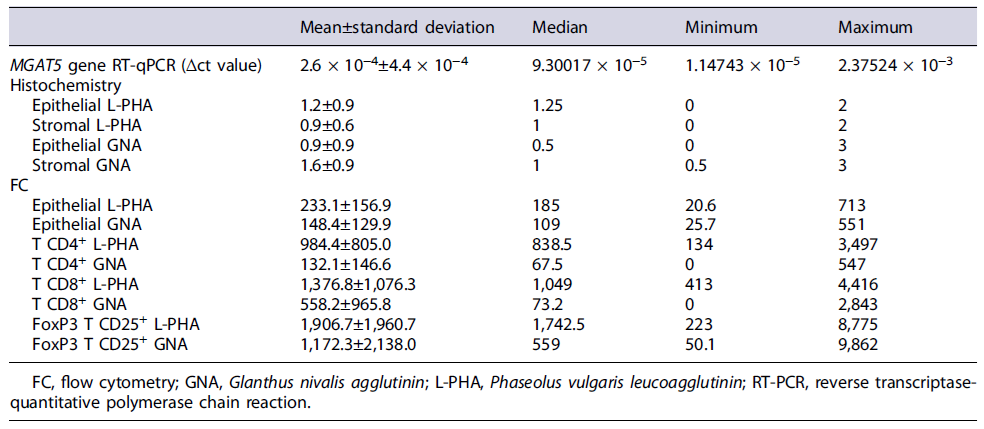
Of the 53 colonoscopies performed, 63 samples of SPL were obtained (34 FFPE biopsies and 29 fresh biopsies). This cohort includes 44 (69.8%) SSL without dysplasia, 9 (14.3%) SSL-D, 7 (11.1%) HP, 1 (1.6%) adenocarcinoma of the serrated pathway, and 2 (3.2%) SSL-D with concomitant adenocarcinoma (Table 1). The description of the sample regarding the remaining sociodemographic characteristics, risk factors, and anatomopathological characteristics is depicted in detail in Table 1. The descriptive statistic of N-glycosylation profile is depicted in detail in Table 2.
Altered Premalignant Epithelial N-Glycosylation Profile Is Correlated with Age, Smoking, Increased BMI, Polyp’s Dimensions, and Lesion Location in the Serrated Pathway.
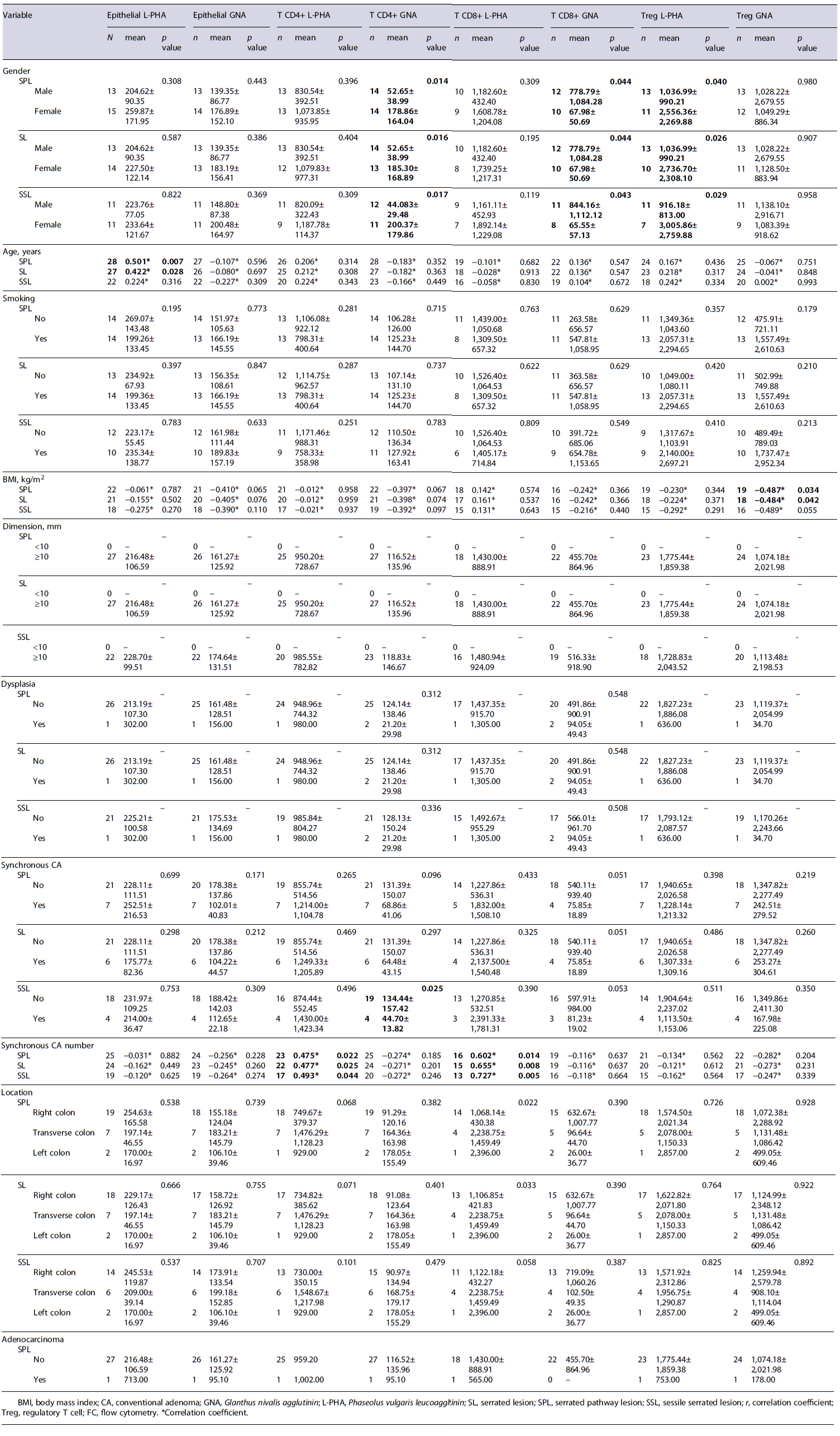
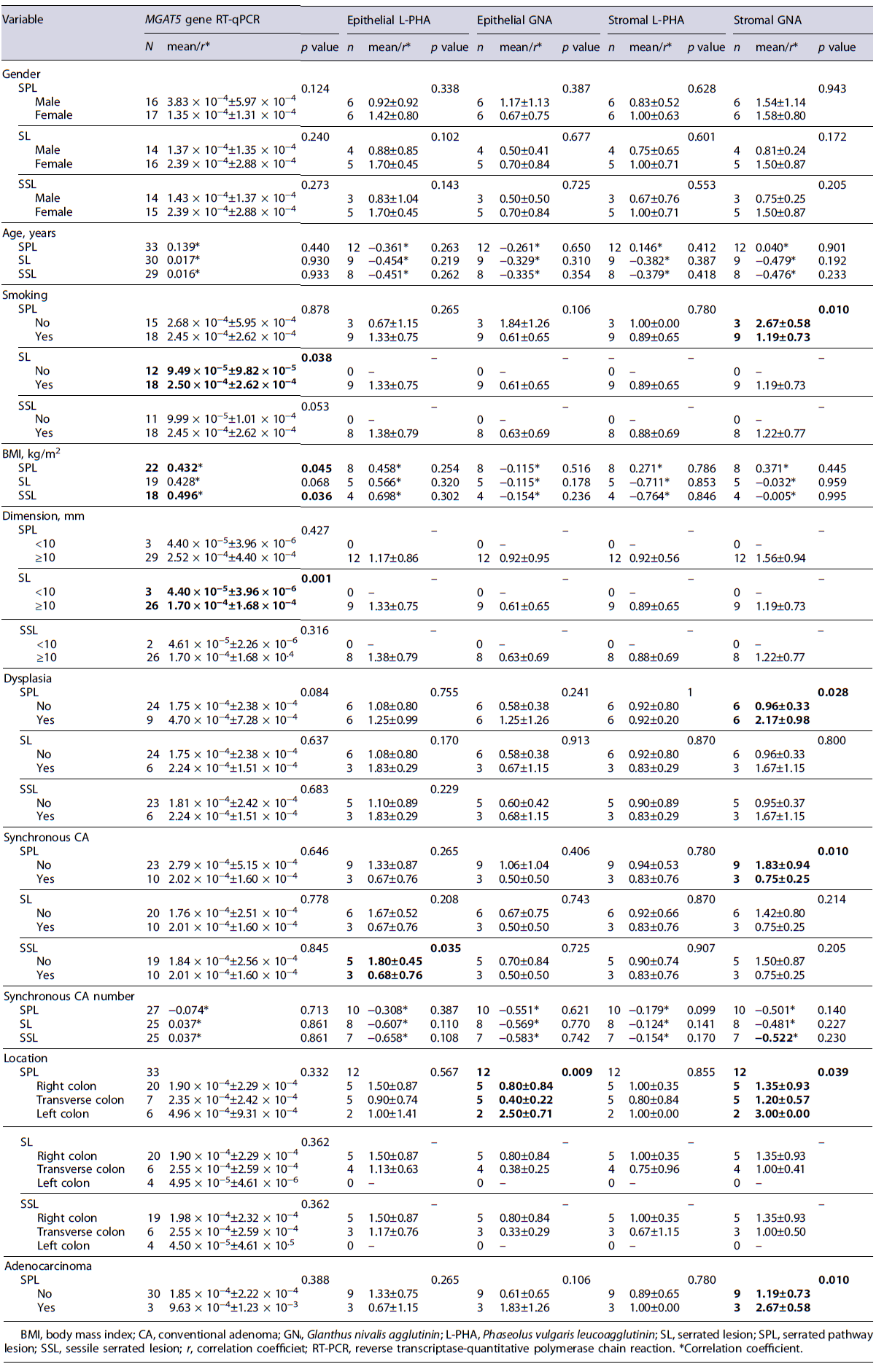
The N-glycosylation profile of epithelial cells was correlated with potential risk factors for disease progression in the serrated pathway. Our results indicated a significant correlation between increased age and the β1,6-GlcNAc branched N-glycans expression (L-PHA expression) on epithelial cells in SPL (p = 0.007; r = 0.501)(Table 3). Additionally, tobacco consumption was also associated with an increased MGAT5 gene expression. In fact, smokers presented an evident higher β1,6-GlcNAc branched N-glycans expression in SL comparing to non-smokers (Δct value: 2.50 × 10−4 ± 2.62 × 10−4 vs. 9.49 × 10−5 ± 9.82 × 10−5; p = 0.038) (Table 4). Furthermore, a statistically significant correlation was also observed between body mass index (BMI) and β1,6-GlcNAc branched N-glycans expression in SPL (p = 0.045; r = 0.432) and in SSL (p = 0.036; r = 0.496), regarding MGAT5 gene expression (Table 4). In addition, our data indicate a higher β1,6-GlcNAc branched N-glycans ex-pression in SL with dimensions ≥10 mm, comparing to SL with dimensions <10 mm, regarding MGAT5 gene expression (Δct value: 1.70 × 10−4 ±1.68× 10−4 vs 4.40 × 10−5 ±3.96× 10−6; p = 0.001) (Table 4). Concerning the SPLs location, there was an increase in the high-mannose N-glycans expression in the epithelial part of the left colon, compared to the right and transverse colon (2.50 ± 0.71 vs. 0.80 ± 0.84 vs. 0.40 ± 0.22; p =0.009)(Table 4).
Altered Immune N-Glycosylation Profile Is Correlated with Increased Synchronous CA, Sex, Smoking, Location of the Lesion, and BMI in the Serrated Pathway
The N-glycosylation profile of immune cells was correlated with potential risk factors for disease pro-gression in the serrated pathway. Our results demonstrated a positive correlation between the synchronous CA number in patients with SPL, SL, and SSL and β1,6-GlcNAc branched N-glycans expression in CD4+ T cells (p = 0.022, r = 0.475; p = 0.025, r = 0.477; p = 0.044, r = 0.493) and CD8+ T cells (p = 0.014, r = 0.602; p = 0.008, r = 0.655; p = 0.005, r = 0.727)(Table 3). Furthermore, synchronous CA in patients with SPL was associated with a lower high-mannose N-glycans expression in the stromal cells of SPL (0.75 ± 0.25 vs. 1.83 ± 0.94; p = 0.010) and specifically in CD4+ T cells in patients with SSL (44.70 ± 13.82 vs. 134.44 ± 157.42; p = 0.025) (Tables 3, 4). Furthermore, smokers showed, on average, a lower high-mannose N-glycans expression in the SPL stromal cells, when compared with non-smokers (1.19 ± 0.73 vs. 2.67 ± 0.58; p = 0.010) (Table 4). Regarding the BMI, we observed an inverse relationship between this factor and the expression of high-mannose N-glycans in Tregs in SPL and SL (p = 0.034, r = −0.487; p = 0.042, r = −0.484)(Table 3). Additionally, females presented, on average, a higher high-mannose N-glycans expression in CD4+ T cells in SPL (178.86 ± 164.04 vs. 67.98 ± 50.69; p = 0.020), in SSL (299.87 ± 179.86 vs. 44.08 ± 29.48; p = 0.017), and in SL (178.86 ± 164.04 vs. 52.65 ± 38.99; p = 0.016) comparing to males (Table 3). Moreover, females showed, on average, a higher β1,6-GlcNAc branched N-glycans expression on Tregs in SPL (2,556.36 ± 2,269.88 vs. 1,036.99 ± 990.22; p = 0.040), in SL (2,736.70 ± 2,308.10 vs. 1,036.99 ± 990.21; p = 0.026), and in SSL (3,005.86 ± 2,759.88 vs. 916.18 ± 813.00; p = 0.029), comparing with males (Table 3). On the other hand, males presented, on average, a higher high-mannose N-glycans expression in CD8+ T cells in SPL (778.79 ± 1,084.28 vs. 67.98 ± 50.69; p =0.044),in SSL (884.16 ± 1,112.12 vs. 65.55 ± 57.13; p = 0.043), and in SL (778.86 ± 1,084.28 vs. 67.78 ± 50.69; p = 0.044) (Table 3). Furthermore, left colon lesions presented a higher high-mannose N-glycans expression in stromal cells compared to the transverse colon (3.00 ± 0.00 vs. 1.20 ± 0.57; p =0.039)(Table4). SPLwith dysplasia showed a higher high-mannose N-glycans expression in stromal cells (2.17 ± 0.98 vs. 0.96 ± 0.33; p = 0.028), comparing with non-dysplastic SPL (Table 4). Also, in serrated pathway adenocarcinomas and SSL-D with concomitant adenocarcinoma a higher high-mannose N-glycans expression on stroma was observed on average (2.67 ± 0.80 vs. 1.19 ± 0.73; p = 0.010) (Table 4).
Discussion
SPL follow-up still raises serious concerns due to rapid progression from dysplasia to cancer. Moreover, there are few robust studies of clinical progression risk factors on serrated pathway. The changes in N-glycosylation has been considered as a CRC progression hallmark in epithelial cells [11]. Thus, the main goal of our study was to define potential risk factors for progression to malignancy by analyzing the N-glycosylation profile of the serrated pathway.
We found an increased β1,6-GlcNAc branched N-glycans expression in the SPL epithelial component correlated with increasing age and BMI. In SL, β1,6-GlcNAc branched N-glycans expression in the epithelial component is also correlated with smoking and polyp dimensions ≥10 mm. Previously, our group showed that these types of glycans present an aberrant expression in CRC and are direct immune modulators in the tumor microenvironment, allowing immune evasion [14]. Thus, these clinical variables may intervene as risk factors for the progression to malignancy in the serrated pathway by enabling identification of immunological escape in these lesions. In fact, age is a known risk factor for the CRC development and progression, with immunosenescence potentially playing an important role in the immune escape suggested in our results [1, 17]. Smoking is a studied and validated risk factor for SL development and progression to CRC [17, 18]. This evidence is in line with our results, emphasizing smoking cessation as a method of preventing progression in the serrated pathway. Additionally, BMI has been described in the literature as a possible risk factor for CRC progression [19]. However, it is not fully understood whether it impacts the adenomacarcinoma pathway or the serrated pathway [19]. Our findings suggest that BMI may contribute to progression to malignancy in the serrated pathway by upregulating β1,6-GlcNAc branched N-glycans in epithelial cells. Thereby, weight loss should be encouraged in individuals with SPL in an attempt to prevent progression to malignancy. Regarding SL dimensions, our results suggest that polyps with dimensions ≥10 mm have greater risk of progression to malignancy, by overexpression of β1,6-GlcNAc branched N-glycans in epithelial cells. This result is in line with the European guideline for post-polypectomy colonoscopy follow-up, which defined a cut-off of 10mm to perform a shorter interval follow-up [20].
Regarding the immune compartment, it was observed a higher β1,6-GlcNAc branched N-glycans expression with the increasing number of synchronous CA in patients with SPL. Previously, our group had shown that an increasing β1,6-GlcNAc branched N-glycans expression in T cells, by GlcNAc supplementation, controls T-cell immune response in inflammatory bowel disease, by increasing its threshold of activation [16]. Thus, T-cell β1,6-GlcNAc branched N-glycosylation seems to create an immunosuppressive environment disabling T-cell activation and function in SPL, promoting their growth. This immunosuppression promotes the development of more synchronous CA. Therefore, a higher synchronous CA number seems to be a risk factor for progression to malignancy in the serrated pathway. Synchronous CA number has not been described in the literature as a risk factor for development or progression to malignancy in the serrated pathway yet. Thereby, the anatomopathological identification of the N-glycosylation pattern in the presence of a high syn-chronous CA number can select patients that would benefit from a shorter interval of follow-up, to reduce interval cancers. However, we did not distinguish the dysplasia type of CA (high-grade vs. low-grade dysplasia), which may limit these conclusions.
According to our data, smoking and synchronous CA presence seem to be risk factors for progression to CRC in the serrated pathway, since they are correlated with high-mannose N-glycans downregulation on T cells, suggesting decreased immune function in SPL. Furthermore, it seems that the lower immune system activation by downregulation of high-mannose N-glycans enables the synchronous CA development. In fact, Li et al. [21] verified that the synchronous CA presence in patients with SL confers an increased risk of developing CRC. Regarding this, our results are in accordance with the literature, emphasizing N-glycosylation as a new potential biomarker for malignancy progression in serrated pathway. Contrariwise, we also showed an increased stromal high-mannose N-glycans expression in the presence of dysplasia and adenocarcinoma with concomitant SSL-D and in the left colon lesions. These findings suggest that the immune system is more active both in the presence of dysplasia and adenocarcinoma of the serrated pathway, contradicting what we expected. We predicted a reduced high-mannose N-glycans in immune cells related with T cells inability to recognize neoplastic lesions. Thereby, we need further investigations with a larger sample size to clarify these results. Regarding SPL location, left colon lesions appear to have a more active immunological profile, by having a higher high-mannose N-glycans expression, suggesting that this location is less likely to progress to CRC. In fact, SPL is more frequent in the right colon [22]. These results have a follow-up impact, considering the low potential for progression of a left colon lesion. Therefore, the N-glycosylation profile in colonic immune compartment could be used to guide clinicians on follow-up decision.
As previously mentioned, we found an association between the β1,6-GlcNAc branched N-glycans in epithelial component and a high BMI. An increased BMI was also associated with a reduced high-mannose N-glycans expression on Tregs. These results suggest that Tregs might have an increased immunosuppressive capacity in the presence of elevated BMI, creating an immunosuppressive microenvironment, which contributes to progression to malignancy. Thus, as emphasized earlier, patients with higher BMI may be considered for a closer surveillance of SPL.
Regarding gender, a different N-glycosylation pattern was observed in different immune system cells. In fact, β1,6-GlcNAc N-glycosylation in different genders has never been studied before. Our results showed a higher high-mannose N-glycosylation expression in CD4+ T cells in females and in CD8+ T cells in males in all serrated pathway, highlighting that men and women have higher activation of different T cells. Both CD8+ and CD4+ T cells play a role in tumor eradication by direct action (CD8+ T cells) and cytokine release (CD4+ T cells)[12]. Despite this, CD8+ T cells infiltration in tumor microenvironment is correlated with a better prognosis in CRC, suggesting that men have lower progression to cancer in serrated pathway [23]. By opposition, Tregs in females have a higher β1,6-GlcNAc branched N-glycans expression creating an immunosuppressive environment that allows the progression to malignancy. Regarding this, our results suggest a different N-glycosylation pattern of immune system cells occurring in both genders, which demands further investigation.
To conclude, biomarkers are an essential tool in current medical practice, playing an important role on understanding and identifying several diseases. Our study showed that N-glycosylation could be a potential biomarker of tumor progression in the serrated pathway. This study set the ground for the potential inclusion of β1,6-GlcNAc branched N-glycans and high-mannose N-glycans in the SPL anatomopathological analysis to select those patients who need shorter intervals of follow-up to reduce the interval cancers incidence. Furthermore, according to N-glycosylation profile, we identified smoking, aging, elevated BMI, SL dimensions ≥10 mm, the presence and number of synchronous CA as risk factors to progression to malignancy. These associations allow directed clinical interventions based on risk factors to reduce the progression to malignancy. Taken together, N-glycosylation profile seems tobeone keytosolve this puzzling pathway with large impact in clinical and molecular research. Despite our results, this study has several limitations, namely, the small sample size, the high missing data value, and the lack of similar articles that prevent us to draw more conclusions.