Introduction
Short bowel syndrome (SBS) is a malabsorptive condition that results from the loss of intestinal length due to disease or resection [1]. SBS is the most common cause of chronic intestinal failure (CIF) [2]. Due to the major loss of digestive and absorptive surface area, general consequences of CIF include diarrhea, dehydration, electrolyte abnormalities, and weight loss. Patients need parenteral support for months or years, parenteral nutrition (PN), or hydration/electrolyte supplementation. For decades, Portuguese CIF patients have been confronted with major difficulties in receiving home parenteral nutrition (HPN)[3, 4]. In 2020, a new regulation, “Norma 017/2020” [5], set the conditions for home CIF management, and more patients are being treated in an ambulatory setting. We aimed to review the general concepts, definitions, and classifications of CIF-SBS in adults, as well as to address the multimodal treatment of these patients and disease/HPN-related complications.
Concepts, Definitions, and Classification of Intestinal Failure in Adults
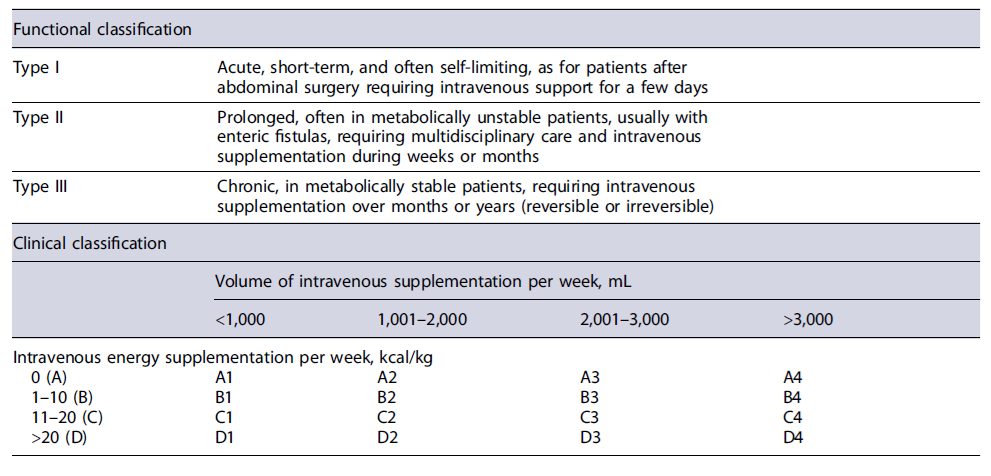
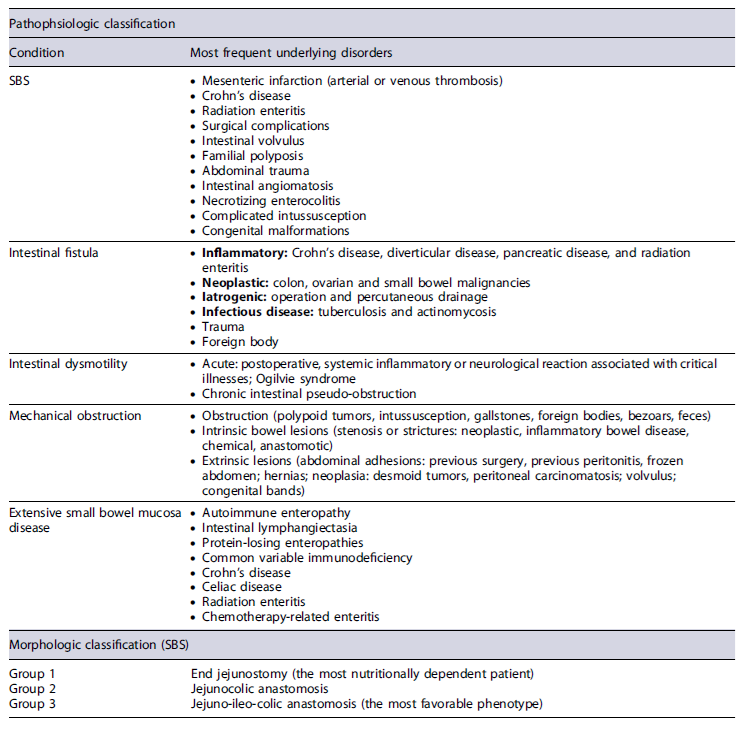
Intestinal failure (IF) is defined as a reduction in gut function below the minimum necessary for absorption of macronutrients and/or water and electrolytes. Intravenous supplementation is required to maintain health, growth, and body homeostasis. The reduction in absorption that does not require intravenous supplementation is called intestinal insufficiency [2]. Several classification criteria were used [2, 6-10] to reflect the different perspectives (Tables 1, 2).
Table 2 Pathophysiologic and morphological classifications for intestinal failure and short bowel syndrome
From a clinical point of view, IF definition implies the necessity of intravenous support [2]. IF should be anticipated in patients with (1) a jejunostomy/ileostomy and <200 cm of proximal small bowel, (2) <100 cm of small bowel and colon in continuity, and (3) a stoma or fistula output >1.5 L/day [7]. The need for intravenous support may depend on the effectiveness of oral nutrition/hydration, quality of oral intake, and use of specific pharmacotherapy. In borderline patients, intestinal insufficiency or IF may depend on the quality of clinical management and intestinal rehabilitation.
Multimodal Treatment of CIF-SBS and Intestinal Rehabilitation
Management of CIF-SBS aims to reduce gastrointestinal secretions, slow transit, correct/prevent malnutrition, dehydration, and specific nutrient deficiencies, and to prevent refeeding syndrome [11]. Once these early targets are achieved, progression to a stable nutritional regimen is required as part of the intestinal rehabilitation process. The need for intravenous fluid/nutrition is dictated mainly by the cause of CIF, anatomy of the SBS, and pathophysiological consequences [12].
Fluid Support and Management of Dehydration in SBS
Patients with SBS are prone to dehydration [13, 14], as they are net secretors, loose fluid, and sodium from the intestine. Since the primary deficit is not chloride, which is less well cleared from the body, a balanced electrolyte solution such as Ringer’s lactate is preferred instead of saline solution [13].
Gastrointestinal losses should not dictate an increase in oral liquid intake because in SBS, this leads to fluid secretion into the proximal intestine, increasing losses. Urine output is useful for monitoring dehydration in SBS, and once an acceptable volume (20 mL/kg per 24 h) has been achieved, a transition to an oral rehydration solution (ORS) may be considered, as well as a combination of oral/enteral and parenteral approaches [7]. The importance of restricting the intake of low-sodium fluids, such as hypotonic fluids (e.g., water, tea, alcohol, and coffee) and hypertonic fluids (e.g., regular soda and fruit juices), should be emphasized. Instead, an ORS to enhance absorption and reduce secretion is preferred in patients with an end jejunostomy [15]. ORS is rarely needed in SBS with colon in continuity because patients usually maintain adequate hydration.
Oral Nutrition
Dietary therapy should focus on the maintenance of compensatory hyperphagia. Even small amounts of luminal nutrition stimulate intestinal adaptation and protect against liver diseases and other complications [7]. Dietary counseling should be based on patient preferences to ensure high compliance. Adjustments can be made based on the tolerance, symptoms, stool output, and weight. Due to malabsorption, dietary intake must be increased by at least 50% from the estimated needs, divided into 5-6 meals throughout the day [16]. High-energy-density foods with high salt content are recommended. Patients should use salt liberally and restrict oral fluid intake during meals.
In patients with SBS and colon in continuity, a high-carbohydrate (60%), low-fat (20%) diet with oxalate restriction (e.g., peanuts and baked beans) tend to reduce fecal calorie loss, increase energy absorption, and reduce magnesium/calcium and oxalate absorption. A high content of medium-chain triglycerides should be suggested, as well as fat restriction, as it results in steatorrhea and reduces carbohydrate fermentation [13, 17]. The fat/carbohydrate ratio in patients with end jejun ostomy is less important;as enteral fat is useful owing to its energy density, they do not benefitfromits restriction [18, 19].
Enteral Nutrition
Enteral nutrition (EN) should be considered, especially in those with low PN dependence, who are expected to be weaned off. Even in patients with a limited potential for complete PN weaning, EN can achieve considerable benefits [6].
Considering the altered anatomy and intra-abdominal adhesions, frequent in SBS, performing a percutaneous gastrostomy can be technically challenging. The risks and benefits must always be discussed, and a trial with a nasogastric tube should be performed.
Polymeric formulas are preferred over elemental formulas because they are less costly, less hyperosmotic, and well tolerated. However, studies suggest that both formulas are similar in terms of nutrient absorption and fluid/electrolyte loss. Continuous infusion seems to en-hance the benefits and tolerance of EN. Overnight feeding improves quality of life and enables normal daily activities [20].
Home Parenteral Nutrition
Indications and Aims
HPN is indicated in patients who cannot meet their nutritional requirements despite maximal medical therapy, including oral/EN and who can be safely managed outside the hospital. It aims to support nutrition, provide hydration, and avoid electrolyte disturbance. HPN also promotes autonomy and a better quality of life, as well as intestinal rehabilitation, as part of the desired weaning off process [6].
Training and Monitoring
Before starting HPN, the patient must be metabolically stable, able to cope with HPN therapy, and have adequate social support and home environment. Patients and/or caregivers must demonstrate self-care competency before discharge [7]. Training starts during hospitalization and aims to ensure the safe practice of HPN by teaching all aspects of infusion. Catheter care and pump use should be focused on to prevent, recognize, and effectively manage possible complications. Despite its recognized importance, there are no available guidelines for training patients/caregivers [6]. The European Society for Clinical Nutrition and Metabolism (ESPEN) encourages HPN patients to join nonprofit groups that can assist in providing education, support, and networking, which are beneficial in terms of QoL, depression scores, and catheter infections [7].
Regular contact between the nutritional support team (NST) and the patient is essential, initially every few days, then weekly, and eventually monthly. Monitoring weight, urine/stoma output, and hydration status is of utmost importance. Serum electrolytes, including sodium, potassium, chloride, bicarbonate, and renal function tests, should be measured frequently until stable and then at regular intervals, monthly to every 3 to 6 months, on a case-by-case basis. Moreover, regular monitoring of chloride and acid-base status through arterial blood samples is recommended [7]. Blood counts, liver enzymes, bilirubin, and albumin levels should also be monitored to address potential complications. Vitamins and trace elements should be measured every 6-12 months. Patients starting HPN should undergo bone mineral densitometry and measurement of markers of bone turnover, such as PTH and vitamin D, at yearly intervals. Biochemistry and anthropometry must be evaluated during all visits.
Quality of care is measured by evaluating HPN-related complications, hospital readmissions, and weight change, as well as by regular audits of the patient’s quality of life. The HPN-QoL is a specific questionnaire [21] that focuses on physical, emotional, and symptomatic issues, although it has not been validated in the Portuguese population.
Components of HPN
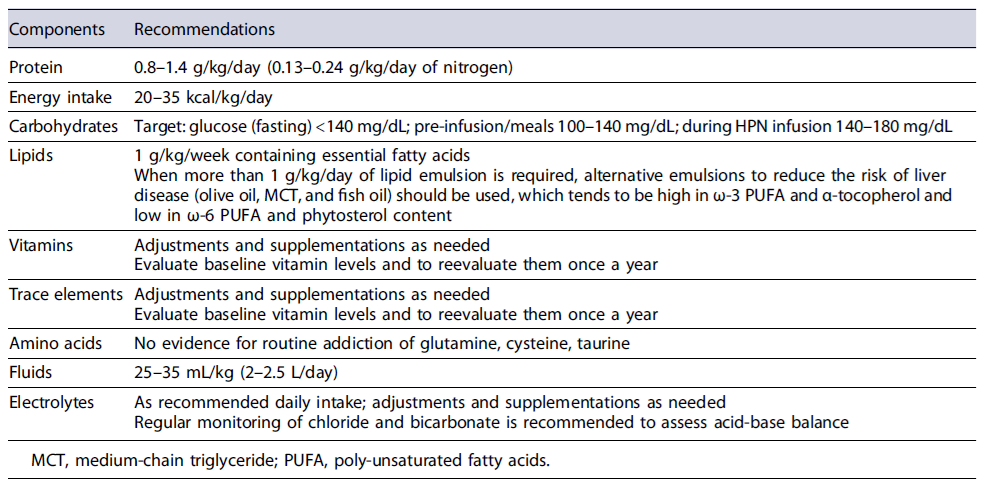
Table 3 summarizes the components of HPN [22-28]. The adequacy of HPN volume should be assessed by 24-h urine output and serial measurements of sodium, potassium, phosphate, magnesium, and calcium levels.
Venous Catheters
Patients with HPN require long-term central venous catheter (CVC). Current guidelines recommend ultrasound-guided catheter placement in a central vein (subclavian or jugular) by an experienced physician to reduce the number of immediate and late complications [7].
Essentially, 2 types of catheters are used for HPN: cuffed tunneled central catheter (Hickman-Broviac) and a totally implanted port catheter (Implantofix-type). The choice between them depends on the frequency of venous access required, patient compliance, and experience of the NST [6, 7, 29]. A Hickman-Broviac is usually preferred, while port catheters are reserved for patients who only need parenteral hydration, who do not use the CVC daily, or who practice sports/other activities that benefit from the CVC totally implanted under the skin.
General recommendations and precautions include [7]:(1) using a single-lumen catheter; (2) choosing the maximum necessary diameter of the catheter for the type of solute to be administered; (3) using the CVC only for the administration of PN; (4) using a perfusion pump for the solute to be administered;(5) handling of the CVC by the same person and monitored by the NST; (6) performing hand hygiene and disinfection before and after handling the CVC; (7) replacing the administration system every 24 h; and (8) flushing the CVC before and after its use with saline solution.
Weaning Perspectives/Enteral Autonomy
Virtually all patients with SBS will require PN, although more than 50% will be able to be weaned completely from PN in 5 years, in parallel with progressive enteral autonomy [10]. However, rehabilitation should be initiated as soon as possible. The probability of eliminating HPN dependence is <6% if not accomplished in the first 2 years [9]. In this matter, two principles apply: avoiding exclusive or total intravenous feeding and implementing oral/EN.
Permanent IF is expected for 100 cm or less of the small intestine in patients with end jejunostomy, 65 cm of jejunum in jejunocolic anastomosis, and 35 cm of small bowel in jejunoileal anastomosis [30]. Patients with colon in continuity and initially less dependent on HPN have generally a better prognosis [31].
HPN Complications
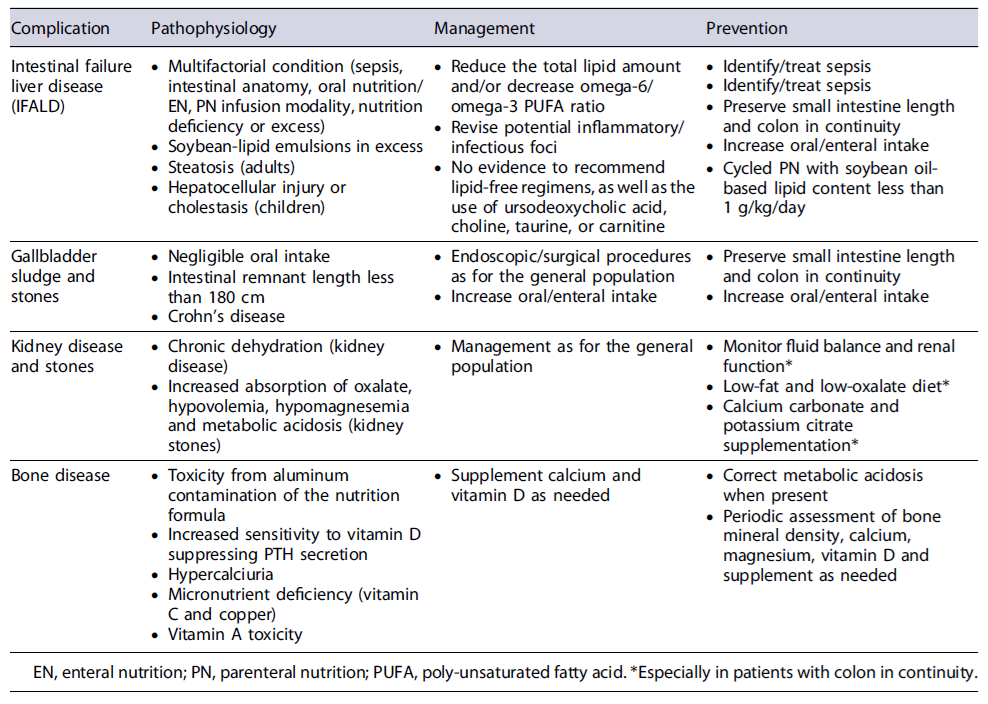
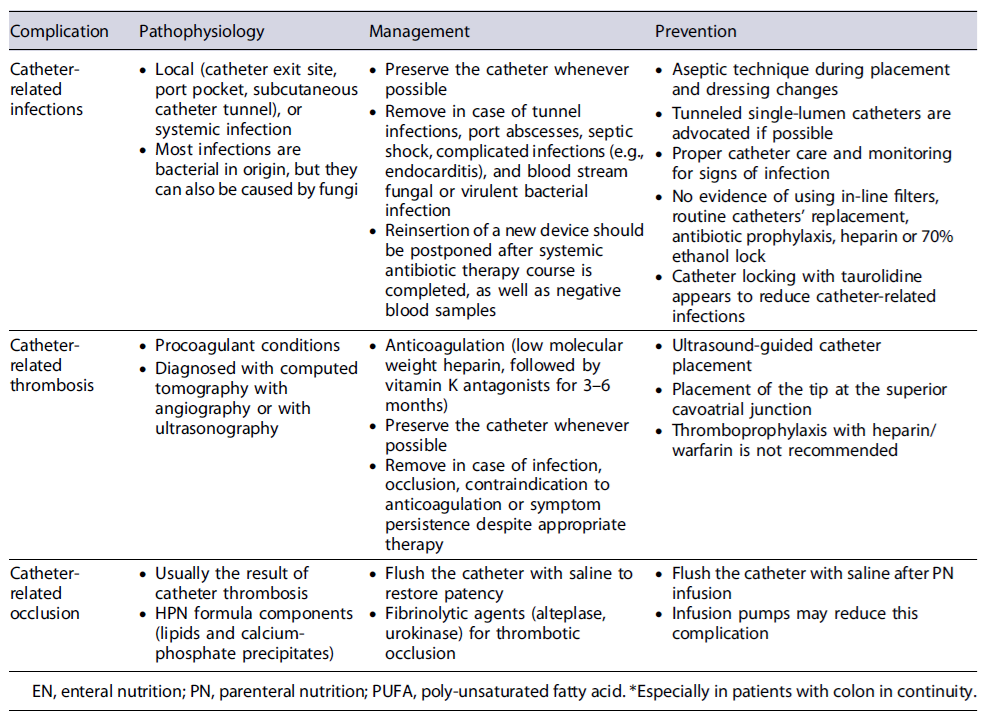
Tables 4 and 5 summarize the disease- and catheter-related complications of HPN [32-41].
Precautions for Orally Administered Medications Absorption of oral medication may be impaired in patients with SBS, especially in those without the proximal jejunum. Entericcoated and delayed-release medications may not be properly absorbed and should be avoided. When feasible, alternative routes (e.g., intravenous, subcutaneous, transdermal, and rectal) may be considered.
Pharmacologic Treatment
Antisecretory Drugs
Enterectomy is associated with gastric hypersecretion and hypergastrinemia, especially within the first 6-12 months after resection, contributing to increased intestinal fluid loss and risk of peptic ulcer disease [42]. Antisecretory drugs, including proton pump inhibitors (PPI) and histamine-2 receptor antagonists, can be used to counteract these effects.
However, these medications increase the risk of small-intestinal bacterial overgrowth. The beneficial effects on stool volume and dyspeptic symptoms should be weighed against this potential risk. The duration of its beneficial effects remains unclear; however, these medications should be used cautiously beyond 6-12 months. ESPEN recommends its use, especially during the first 6 months after surgery, mainly in patients with SBS with a fecal output exceeding 2 L/day, and suggests that these drugs are also effective in reducing fecal wet weight and sodium excretion in the long-term [7, 43].
Antidiarrheal Drugs
Antidiarrheal agents, such as loperamide and codeine, are used to prolong intestinal transit time, enhance absorption, and reduce fecal wet weight and sodium excretion in patients with SBS with ostomy. Since opiate drugs have central nervous system side effects, such as sedation, and may have potential for addiction, loperamide should be preferred. Nevertheless, if necessary, combining these agents can enhance their effectiveness [44]. The use of these agents should be guided by objective measurement of their effects.
High doses of loperamide are frequently needed (reaching up to 32-64 mg/day), especially in patients with SBS without ileum, as it needs to enter the enterohepatic circulation. Although higher than the recommended label dose, loperamide is well tolerated by patients with SBS [45]. Nevertheless, side effects, such as arrhythmias, should be carefully monitored when administered at such doses. Administration 30-60 min before meals and at bedtime is often suggested, although there is no robust evidence for these recommendations.
Octreotide
Octreotide should be considered for patients experiencing severe fluid loss that cannot be effectively managed using conventional treatment. Typical candidates for this therapy include patients with SBS and high output end jejunostomy [46]. Dosage is 100-300 μg subcutaneously three times per day. Careful monitoring is required because of the potential fluid retention and possible negative influence on the intestinal rehabilitation process with prolonged use.
Antibiotics
Bloating, diarrhea, abdominal discomfort, and bowel dilation should raise suspicion for small-intestinal bacterial overgrowth, and empirical antibiotic treatment should be started accordingly. Frequently used antibiotics in this context include rifaximin, metronidazole, trimethoprim-sulfamethoxazole, and amoxicillin-clavulanic acid [47, 48].
However, routine use of antibiotics in patients with colon in continuity is not recommended because it may reduce the benefit of energy salvage due to bacterial fermentation.
Glucagon-Like Peptide-2 Analogs
In recent years, there has been growing interest in the gut endocrine system [49]. In the context of SBS, there is great interest in the use of growth factors. Targeting the glucagon-like peptide-2 (GLP-2) receptor is a promising therapeutic strategy. GLP-2 is an enteroendocrine peptide that acts through a wide variety of trophic effects to enhance mucosal growth, increase mesenteric blood flow, improve gut barrier function, slow intestinal motility, decrease gastric acid secretion, and regulate inflammatory processes [50-53]. Targeting GLP-2 receptors leads to improved absorption and reduced fluid/electrolyte loss [54].
Teduglutide was the first approved GLP-2 analog for the treatment of patients with SBS. It is a recombinant analog of GLP-2 with a longer half-life than the native peptide, allowing a daily subcutaneous injection (0.05mg/kg/day). Typically, teduglutide is used in stable patients who cannot be weaned from PN despite all other therapeutic strategies. It has been shown to reduce PN requirements and potentially lead to complete weaning off in a subset of patients [55-57]. Additional studies are needed to determine whether intestinal adaptation due to teduglutide is sustained after discontinuation.
As this drug acts as a growth factor, it is contraindicated in patients with active or recent malignancies [58]. Screening with colonoscopy before initiating and during treatment is recommended, although the optimal timing and frequency are unknown. A suggested approach involves annual colonoscopy during the first 2 years, followed by subsequent colonoscopies at a minimum interval of 5 years [59].
Clinical experience with teduglutide suggests that it is generally well tolerated, with most adverse events being mild or moderate in severity. Gastrointestinal symptoms are the most reported adverse events, consistent with the underlying disease conditions and intestinal trophic actions of teduglutide [60]. Injection site reactions, stomal complications, and respiratory tract infections were also frequent.
The attractive physiological effects of GLP-2 have prompted many efforts to slow the very short half-life of the native GLP-2 peptide, enabling its use as a therapeutic agent. Longer-acting GLP-2 analogs such as glepaglutide and apraglutide are currently being studied [49]. Apraglutide is a highly selective and potent GLP-2 receptor agonist with high plasma protein binding and low systemic clearance, resulting in a longer half-life than native GLP-2 peptide and teduglutide. Glepaglutide appears to be less potent and less selective for the GLP-2 receptor than apraglutide. Some studies on apraglutide support a once-weekly subcutaneous dosing regimen, whereas studies on glepaglutide support a once- or twice-weekly subcutaneous dosing regimen, which can improve quality of life and treatment compliance [61-64].
Glucagon-Like Peptide-1 Analogs
GLP-1 analogs (e.g., exenatide, liraglutide, dulaglutide, or semaglutide) have been used for almost 2 decades in the treatment of diabetes mellitus type 2 and, recently, in obesity. Targeting the GLP-1 receptor in patients with SBS may influence proximal gut transit since GLP-1 seems to lack the intestinal trophic properties of GLP-2. Some studies have shown a reduction in ostomy output and a reduction in PN requirements with GLP-1 analogs [65-67]. Another study evaluated the effects of continuous infusion of GLP-1, GLP-2, and a combination of both (GLP-1 and GLP-2) in adults with SBS. The authors found that all treatments significantly reduced fecal wet weight compared to the placebo. The effects of GLP-1 were less potent than those of GLP-2, but the combination therapy was shown to have superior efficacy compared to each single agent, further supporting the rationale for a GLP-1/GLP-2 combination strategy in SBS [67].
Growth Hormone (Somatotropin)
The use of growth hormone (GH) in SBS patients showed a moderately favorable effect on intestinal wet weight loss, even though its use could be associated with significant side effects such as peripheral edema, arthralgia, and carpal tunnel syndrome. There are also concerns about the potential increased risk of diabetes mellitus and cancer [47]. The positive effects of GH have mainly been described in patients with SBS with colon in continuity. GH is approved only in the USA and its role is being replaced by GLP-2 agonists.
Clonidine
Clonidine has demonstrated some benefits in treating high output stool losses, likely due to its effects on intestinal motility and secretion [68, 69]. However, further studies are required to better understand its effectiveness.
Bile Acid Binders and Pancreatic Enzymes
Given the already diminished bile acid pool in patients with SBS, the use of bile acid sequestrants (such as cholestyramine) may exacerbate steatorrhea and fat-soluble vitamin losses and, therefore, should generally be avoided. There is still insufficient evidence supporting the efficacy of pancreatic enzyme supplementation for the treatment of SBS [7].
Surgical Prevention and Treatment of SBS
How to Avoid SBS and IF?
The role of surgery in IF often begins with prevention; therefore, early recognition of patients at a high risk for loss of critical bowel length should trigger conservative strategies [12, 70]. Operative prehabilitation of patients at risk (e.g., inflammatory bowel disease, malignancy, immunosuppression, and significant comorbidities) is indicated and aims to reduce the risk of postoperative complications. Nutritional and comorbidity optimization reduces the risk of high output stomas or the development of enterocutaneous fistulas.
In patients with Crohn’s, bowel-sparing techniques (stricturoplasties) should be selected whenever possible, and wide anastomoses should be constructed to avoid stenosis recurrence. Extended bowel resection should be avoided in patients undergoing emergency surgery (ischemia or perforation). In situations of doubt regarding the viability of segments contiguous to those in the acute process, a “clip and drop” approach [11] should be chosen for damage control, laparostomy, and closure of the abdominal wall postponed until the patient is stable. This approach avoids wide resections and often recruits bowel segments with questionable vascularization in the context of shock, allowing anastomosis creation when conditions become favorable. Critically ill patients should maintain postoperative intra-abdominal pressure monitoring to prevent bowel ischemia.
When to Reoperate?
Patients with type 2 IF may be able to reestablish intestinal continuity; however, they should be assessed preoperatively, and the risks and benefits of new complications should be considered. The decision for reintervention requires knowledge of the disease origin, remanent bowel length, and other anatomic features, such as the presence of stomas, entero-atmospheric fistulas, and blind loops [71]. In this regard, Lal et al. [72] proposed a strategy that includes investigation/treatment of sepsis, assessment/optimization of nutritional status, knowledge of intestinal anatomy, and a long-term plan for each patient. This therapeutic plan was termed the “Sepsis-Nutrition-Anatomy-Plan” (SNAP), which serves as a useful guide to manage patients with type 2 IF.
The timing for reintervention should never be less than 6 months [12, 70, 71] after the last surgery. Procedure-related mortality and recurrence of intra-abdominal complications decrease when the patient is optimized and free of septic complications before the second intervention, which is often performed 9-12 months after the first [11, 73].
During this “bridge-to-surgery period,” it is also important to provide psychological support. Whenever possible, it is recommended that these patients are discharged and receive ambulatory nutritional support before reintervention [11].
Preoperative evaluation requires radiological and endoscopic assessment of the entire digestive tract, including the segments distal to future anastomosis, to exclude stenosis. Abdominal/pelvic computed tomography with oral/vascular contrast is mandatory as an anatomical roadmap of segments to be anastomosed, to exclude intra-abdominal collections, and to evaluate the integrity of the abdominal wall [12]. Enterography and/or pelvic magnetic resonance imaging is indicated in patients with Crohn’s disease to detect enteroenteric fistulas and perianal disease. In the presence of an ileal conduit or in patients who have undergone complex urological procedures, the urinary anatomy should be studied, as it may be beneficial to place ureteral stents preoperatively. There are indirect clinical signs that the abdomen has matured for surgery: absence of visible granulation tissue in the abdominal scars, bowel prolapse through the stoma or fistula orifice and a favorable “pinch test” (when the skin can be pinched and lifted from the underlying bowel, it means it can be easily dissected from it) [73].
During the intervention, technical care should be taken to avoid postoperative complications [74]: (1) complete adhesiolysis of bowel segments; (2) repair of all desperitonization lesions; (3) avoid unnecessary additional resections; (4) prioritize meticulous and manual anastomosis; (5) avoid contact of the anastomoses with the abdominal wall; (6) remove previously placed infected/contaminated abdominal wall meshes; (7) never use a synthetic mesh for reconstruction; and (8) avoid reinterventions in suspected postoperative complications in favor of noninvasive/percutaneous procedures.
Role of Autologous Intestinal Reconstruction Surgery
In patients who reach a plateau of dependence on PN, with optimized nutritional/pharmacologic treatment and still no progression to enteral autonomy, autologous intestinal reconstruction surgery can be considered to improve intestinal absorption and facilitate the intestinal rehabilitation process [75]. Commonly performed procedures include antiperistaltic reversed segments, colonic interposition, tapering, longitudinal/spiral intestinal lengthening, serial transverse enteroplasty, and controlled tissue expansion. The main goals of autologous intestinal reconstruction surgery include [76] (1) slow intestinal transit to increase contact time between nutrients and mucosa, (2) correct short bowel stasis, (3) improve intestinal motility, and (4) increase mucosal surface area. Each procedure is designed to achieve one or several targets mentioned above and has its own indications and clinical applications, although evidence regarding which patients will benefit from these procedures and the optimal timing to perform the surgery is still scarce. The choice between the different methods should be individualized.
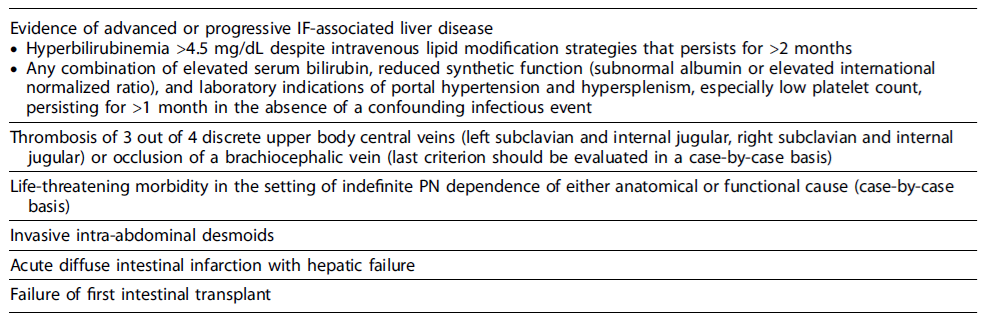
Indications for Intestinal Transplantation Treatment options for irreversible CIF include lifelong HPN or intestinal transplantation (ITx) [34]. Although HPN is considered the primary treatment for CIF, early referral to intestinal rehabilitation centers with medical and surgical expertise is advised.
Four types of ITx have been described: (1) isolated bowel transplant, (2) combined liver-intestine transplant,(3) modified multivisceral transplant (stomach, jejunum, and ileum with or without the liver), and (4) multivisceral transplant (stomach, pancreas, duodenum, jejunum, and ileum with or without the liver). Classical indications that should prompt the assessment of candidacy for ITx in adults were revised in 2019 and are categorized in Table 6 [77].
Conclusion
CIF requires a comprehensive approach by a multi-disciplinary team. Intestinal rehabilitation involves a multimodal treatment that includes nutritional intervention combined with medical management and, occasionally, surgical strategies that aim to reduce PN/HPN dependence, promote enteral independence, and improve quality of life.