Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portuguese Journal of Public Health
versão impressa ISSN 2504-3137versão On-line ISSN 2504-3145
Port J Public Health vol.37 no.1 Lisboa 2019
https://doi.org/10.1159/000500928
RESEARCH ARTICLE
Primary Pharmaceutical Assistance in a Regional Inter-Agency Committee on Health: Evaluation and Shared Action for Organization
A assistência farmacêutica básica numa Comissão Intergestores Regional: avaliação e ação compartilhada para organização
Maurício Massayuki Nambu, Livia Fernandes Probst, Brunna Verna Castro Gondinho, Antonio Carlos Pereira, Jaqueline Vilela Bulgareli, Karine Laura Cortellazzi, Rosana de Fátima Possobon, Luciane Miranda Guerra
Department of Community Dentistry, Piracicaba Dental School, University of Campinas, Piracicaba, Brazil
ABSTRACT
Introduction: Determining how primary pharmaceutical assistance is structured allows us to establish situational diagnoses and to visualize possible implications for an essential part of the Brazilian Unified Health System. - Methods: We evaluated pharmaceutical services of all municipalities that compose the Regional Inter-Agency Committee on Health of Ourinhos-São Paulo (CIR-Ourinhos-SP) through structure, process, and outcome indicators, and, in a shared way, we proposed actions to qualify the practice. We conducted direct observations of drugs, records, documents, interviews with pharmacists, and visits to health care centers, totaling 12 municipalities, 41 health care centers, and 3 pharmaceutical supply services. - Results: In the thematic workshop carried out with municipal representatives, we found that 68.33% of health care centers and 70% of pharmaceutical supply services met good storage practices. We verified an average of 0.85 pharmacists per unit and the presence of 88.91% of marker drugs. Workshop proposals were sent to managers and pharmacists responsible for pharmaceutical assistance of 12 municipalities. - Conclusions: Most indicators were below the established standards, demonstrating the need for adjustments in the infrastructure and provision of training for professionals
Keywords: Pharmaceutical assistance · Health evaluation · Brazilian Unified Health System · Health management
RESUMO
Introdução: A apuração de como as Assistências Farmacêuticas Básicas (AFB) municipais estão estruturadas permite o delineamento do diagnóstico situacional e visualização de possíveis implicações em parte essencial do Sistema Único de Saúde. Métodos: Avaliamos as AFB de todos os municípios que compõem a Comissão Intergestores Regional de Saúde de Ourinhos-SP (CIR-Ourinhos- SP), por meio de indicadores de estrutura, processo e resultado, e propôs-se, de forma compartilhada, ações para qualificar a prática. Foram realizadas observações diretas em medicamentos, cadastros, documentos, entrevistas com farmacêuticos, visitas às unidades de saúde (US), perfazendo 12 municípios, 41 US e 03 Centrais de Abastecimento Farmacêutico (CAF). Resultados: Na oficina temática realizada com os representantes municipais verificou- se, entre outras coisas, que 68,33% das US e 70% das CAF, cumpriam as boas práticas de armazenamento. Foi encontrada a média de 0,85 farmacêutico por unidade e presença de 88,91% de medicamentos marcadores. As propostas da Oficina foram encaminhadas aos gestores e farmacêuticos responsáveis pelas AFB dos 12 municípios. Conclusões: A maioria dos indicadores ficou abaixo dos padrões estabelecidos, demonstrando a necessidade de adequações na infraestrutura e de oferta de capacitações para as os profissionais.
Palavras Chave: Assistência Farmacêutica · Avaliação em saúde · Sistema Único de Saúde · Gestão em saúde
INTRODUCTION
The importance of pharmaceutical assistance in the Brazilian Unified Health System ( Sistema Único de Saúde – SUS) is due to the promotion of users’ access to drugs and supplies and the role of these services in technological innovation and development and rational drug use 1. Therefore, verifying how municipal primary pharmaceutical assistance is structured in organizational terms, infrastructure, and human resources allows us to establish situational diagnoses and to visualize possible implications for an essential part of the SUS, namely Primary Health Care.
Health policy in Brazil is organized through a single integrated and universal system, the SUS, established by the 1988 Constitution and legitimized by Law No. 8080/90, presenting characteristics of decentralization, regionalization, and social participation. The SUS is structured through health care networks, which aim to provide systemic integration, actions, and health care services with continuous, integral, and humanized assistance, distributed in their health care locations based on different technological densities, from low (Primary Health Care) and intermediate (Secondary Health Care) densities to higher density (Tertiary Health Care). In the SUS, the role of intergovernmental administration is fulfilled by Inter-Agency Committees: Bipartite (CIB), Regional (CIR), and Tripartite (CIT) [2 ,3 ].
Integral therapeutic care, including pharmaceutical care, is defined in Law No. 8080/90 as one of the actions to be performed by the SUS 4. Decentralization and strengthening of pharmaceutical assistance were marked by the extinction of the Medicines Center (former Central de Medicamentos – CEME) in 1997 [5] and later with the National Policy of Pharmaceutical Assistance in 2004 6 . Primary pharmaceutical assistance encompasses all these aspects, involving a level of specific health care, whose drugs are present in the Primary Component of Pharmaceutical Assistance ( Componente Básico de Assistência Farmacêutica – CBAF) 7.
In order for these aspects to be efficiently carried out in Primary Health Care, evaluation must be a constant focus of management, to the extent that it is present in legislation and documents, such as in Administrative Rule No. 1555/13, which regulates the application of financial resources and their implementation in the CBAF within the scope of the SUS, establishing the mandatory presentation of the Annual Management Report for follow-up, monitoring, and evaluation of the application of financial resources transferred between the health care funds 1. The evaluation process also meets the strategies of the World Health Organization (WHO) when, for example, evaluating the availability of essential drugs through indicators of ensured access.
Donabedian 9 proposed an operational didactic provision of indicators for evaluating health care services at three levels: structure, process, and outcome. The technical literature in the field [10 ,11 ] and several studies on the evaluation of pharmaceutical assistance have laid foundations in this sense [12 - 14 ]; those related to structure provide information on the quality of the basic structures, which can be verified within each key component, and are used to assist in the formulation of strategies and for planning interventions to improve the pharmaceutical assistance; those related to process inform the mechanisms and activities of the pharmaceutical assistance policies and supervise some aspects such as: selection of essential drugs, allocation of drugs within the health care budget and the financing sector policy, procedures for hiring, distribution, logistics, and information and continuing education on rational drug use, as well as those related to outcome measure – whether these objectives are being achieved 15 .
Health care evaluation, however, does not make sense if the outcomes are not discussed and analyzed, preferably by the actors involved in the evaluated service or activity. This participatory and shared mechanism is essential to achieve relevant outcomes to the studied reality [16 ,17 ]. Our study evaluated the pharmaceutical assistance of Primary Health Care in municipalities that compose the Regional Inter-Agency Committee on Health of Ourinhos-São Paulo (CIR-Ourinhos-SP), through structure, process, and outcome indicators, and proposed, in a participatory manner, interventions for providing the assistance together with local workers and managers, based on our findings.
Materials and Methods
A pretest was performed, and information was subsequently collected through specific indicators to compose an initial situational diagnosis. Subsequently, a thematic workshop was held involving the 12 municipalities that compose the CIR-Ourinhos-SP, belonging to one of the seventeen Regional Departments of Health ( Departamento Regional de Saúde – DRS) of the State Secretariat of Health of São Paulo/SP ( Secretaria de Estado da Saúde – SES/SP), as we can observe in the flowchart (Fig. 1 ). In the state of São Paulo, the administrative division of SES/SP is done through the DRS, and these are subdivided into Regional Inter-Agency Committees (CIR), such as CIR-Ourinhos-SP, belonging to the DRS-IX, responsible for coordinating activities of the State Secretariat of Health at the regional level and for promoting intersector articulation between municipalities and civil-society organizations.
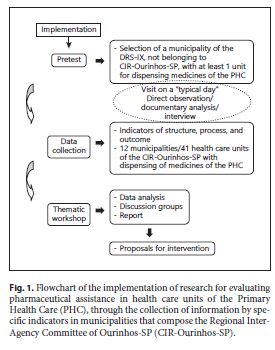
The selection of the study site was based on the position of the studied region in the Social Responsibility Index of São Paulo ( Índice Paulista de Responsabilidade Social – IPRS) 18, selecting a region that presented more than 50% of the municipalities with low levels of income and intermediate levels of longevity and/or education and the most disadvantaged regions of the state, concerning both income and social indicators. This index is used in the state of São Paulo, Brazil, according to the Human Development Index (HDI), which may allow us to compare parameters in future studies, within all geographical limitations and socioeconomic differences that exist in a continental country such as Brazil.
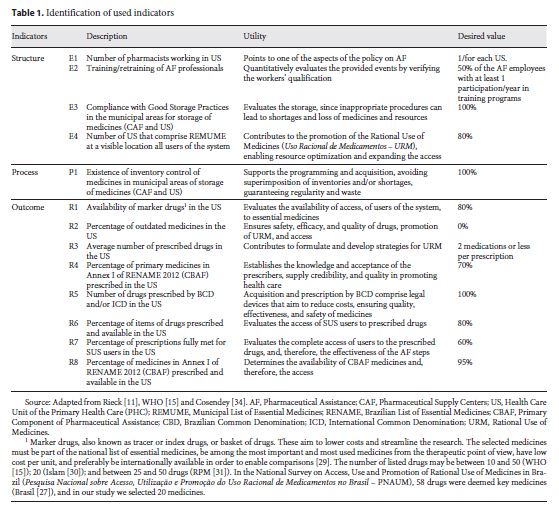
For the outcome indicators R3, R4, R5, R6, R7, and R8 (Table 1 ), 1,230 prescriptions were selected, with 30 prescriptions in each of the 41 studied health care units. This number was based on a previous study by Rieck 11. Such selection was carried out by systematic random sampling, with concealment of the names of the patients present in the unit during the last 5 days prior to the visit. In cases in which the number of prescriptions was unfeasible, the prescriptions from subsequent previous days were selected, up to the number of 30 prescriptions.
Inclusion and Exclusion Criteria
All Primary Health Care Units that comprise the CIR-Ourinhos-SP, which dispensed the drugs part of the CBAF, were included in the study. In the case of prescriptions, those originating from any institution other than Primary Health Care Units were excluded, as well as those identified as unreadable by the researcher and/or unsigned and/or without identification of the legally qualified prescriber.
Drugs considered available were those present in prescriptions and that provided some type of dispensing identification. Prescriptions retained by the health care centers that contained only one drug prescribed without health care identification were deemed available. The drug was considered prescribed by the generic name when its nomenclature was in agreement with the Brazilian Common Denomination (BCD). Prescriptions recorded with a brand name were accepted when the generic name was also properly written. Prescriptions with generic names were accepted when incorrectly written and/or abbreviated, provided they were clearly identified by the researcher and had no registered trademark with their abbreviation, such as AAS®. Existing nomenclatures in the Brazilian List of Essential Medicines ( Relação Nacional de Medicamentos Essenciais – RENAME) and those generally accepted were also considered, such as oral rehydration salts, mineral oil, and zinc paste.
In the case of herbal medicines, we considered the synonymy or the popular nomenclature according to the Form of Herbal Medicines of the Brazilian Pharmacopoeia [19] or Board Resolution No. 95/08 20.
Description of the Indicators
Indicators were identified, according to WHO criteria, in order to provide the following information:
Indicators of Structure (E n ): information on the quality of basic structures of pharmaceutical care
Indicators of Process (P n ): information on the mechanisms and activities of the pharmaceutical care policy
Indicators of Outcome (R n ): information on achieving objectives of pharmaceutical care policy, availability of and accessibility to essential drugs, quality, and rational use
Thematic Workshop
After data collection, a thematic workshop was held with the present municipalities of the CIR-Ourinhos-SP, mainly focused on those responsible for the municipal pharmaceutical assistance. Objectives of the study, results of the survey, and description of the script for conducting the study were presented. There were three groups of four members each, and 80 min to discuss the collected data, reporting causes, proposals, and challenges for facing/improving them, with the presence of the mediator/researcher in each group, in a successive way, stimulating discussions without, however, interfering with the proposals. This was followed by a 60-min plenary session, in which the proposals of each group were presented. The workshop had a total duration of 5 h, with enough space for each group to discuss and list the proposals. Primary pharmaceutical assistance was specially addressed, focusing on the results found in the initial survey of indicators in order to analyze such results, discuss possible causal factors, and develop strategies in a participatory and collaborative manner.
Results
In Table 2 we show the results for each indicator in their respective municipalities, regional average, and comparative standards, according to the literature. The municipalities were identified by twelve capital letters (from A to L) without logical order.
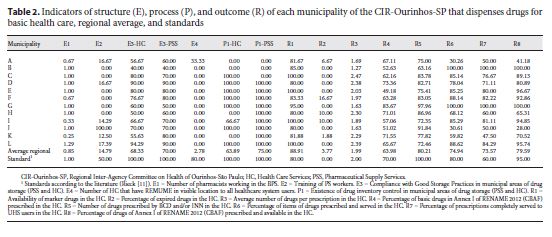
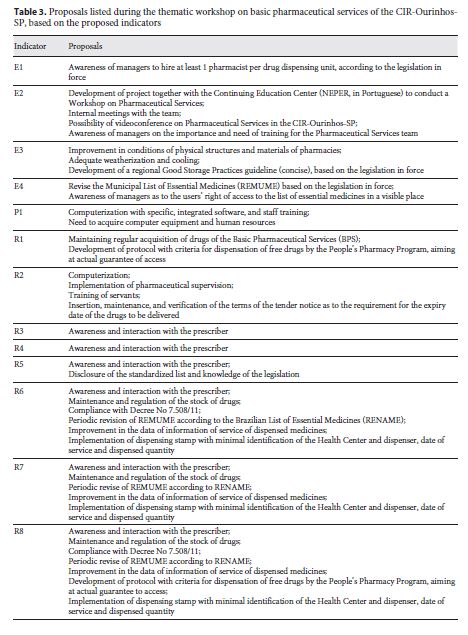
The E3 Indicator of Structure and the P1 Indicator of Process apply both to Pharmaceutical Supply Centers ( Centrais de Abastecimento Farmacêuticos – CAF) and to Health Care Units ( Unidades de Saúde – US), in such a way they are identified as E3-US, E3-CAF, P1-US, and P1-CAF. The thematic workshop was attended by representatives from 7 of the 12 municipalities (58.3%) that compose the CIR-Ourinhos-SP. Specific aspects were discussed in each indicator, and in Table 3 we present the current and consolidated proposals.
Discussion
The novelty of this study is in the creation of local and regional proposals through a participative and democratic process based on the observed situational reality, aiming to achieve goals validated by the literature.
The major debate during the workshop on pharmaceutical assistance conducted in this study concerned the responsibility of the pharmaceutical professionals and the importance of their actions in the resolution of therapeutic treatments. One of the first collective findings was that municipalities need to hire more pharmacists, since the number of pharmacists found in the primary pharmaceutical assistance of the CIR-Ourinhos-SP was below the standard of at least one professional per each dispensing unit 21. In 2005, Naves and Silver [22] showed outcomes even less significant in the Federal District, with 0.13 professionals per US. Bernardi et al.12, when evaluating 20 municipalities of Rio Grande do Sul state, according to the primary pharmaceutical assistance evaluation, found 0.25 professionals per US. However, Menolli et al. [23] and Freitas and Nobre [14] found no pharmacist in 10 health care centers in the municipalities of Londrina (in Paraná state) and Mombaça (in Ceará state). As reported by Naves and Silver 22, drugs that act on the central nervous system, included in ordinance No. 344/98 5, are not dispensed in the absence of a professional in the center, which was also observed in our study. This does not preclude access to essential drugs for mental health; however, it can excessively hinder it.
In addition, with regard to work management, the number of pharmaceutical assistance workers who participated in trainings in the 12 months prior the survey was lower than the standard set by Rieck 11, which is at least 50%. However, Freitas and Nobre 14, in a study conducted in a municipality of Ceará state, reported that there was no training program for pharmaceutical assistance employees. The Brazilian legislation on health care is clear as to the need for the development and training of human resources. According to Law No. 8.080/90, training and continuing education comprise a coordinated intersector action, defining the creation of a permanent committee of integration between health care services and vocational and higher education institutions 4. Training and development of human resources is the guideline of the National Drug Policy (Política Nacional de Medicamentos),and the development, enhancement, appreciation, establishment, and training of human resources [6 ,24 ] is one of the strategic priorities of the National Pharmaceutical Assistance Policy (Política Nacional de Assistência Farmacêutica) . Thus, the development of projects and even immediate practical actions to promote training in the field of Pharmaceutical Assistance is paramount. Therefore, during the workshop, through analysis and discussion of these outcomes, we proposed the formation of a study group with pharmaceutical professionals of the CIR-Ourinhos-SP.
Regarding the indicator that deals with the proper storage of drugs (E3), of the ten checked items, no municipality met the goal of 100%. Barreto and Guimarães 25 , in a study on municipalities in the state of Bahia, detected the absence of physical and environmental conditions for the storage of drugs as well as inadequate transportation and absence of the pharmacist in dispensing. These factors can lead to loss of drugs due to being expired, resulting in a waste of resources and in access to drugs of dubious quality.
A pharmaceutical supply services evaluation study carried out in Brazil in 2004 by the Pan American Health Organization (PAHO) [26] showed overall averages of 61.1% of items met by the health care centers, lower than that found in our study, and 70.1% by the pharmaceutical supply services, virtually identical to the values we obtained.
Regarding good storage practices, in our study, as well as in the studies by Bernardi et al. [12] and Freitas and Nobre 14, we established the same indicator (E3). However, to determine it, it is necessary to verify various items and these items have no standardization, in such a way that the cited studies analyzed several items, although they assessed the same indicator proposed in our study, considering that the checklist for verifying good storage practices is not far from the ultimate goals of the evaluation. It is noteworthy that in our study we did not use the same checklist as PAHO [26] and Barreto and Guimarães 25 , which can be considered a limiting factor. Therefore, in addition to standardization of indicators, we suggest the standardization of items to be deemed indispensable.
Concerning the indicator of structure related to the Municipal List of Essential Medicines ( Relação Municipal de Medicamentos Essenciais – REMUME), established in a visible location to users (E4 indicator), only one health care center of CIR-Ourinhos-SP was in compliance. In a study on pharmaceutical assistance evaluation in Brazil 26 , and in Londrina, in Paraná state, by Menolli et al. 23, 70% of the US had a list of drugs, but did not describe whether they were visible and/or available to users. The same occurred in the National Survey on Access, Use and Promotion of Rational Use of Medicines in Brazil ( Pesquisa Nacional sobre Acesso, Utilização e Promoção do Uso Racional de Medicamentos no Brasil – PNAUM), according to which 85.9% of the municipalities presented a standardized list, and its access by the municipality is available in most units (77.6%), followed by pharmacies (65%) and the municipal department of health (51.3%) 27 .
According to Rieck 11, such is an indicator of political dimension and that gives users information about the stage of their treatment. The reasons for this nondisclosure of the standardization may reflect difficulty or concern of the pharmaceutical care regarding failure to maintain a correct supply of drugs in health care centers, both due to management problems and to scarcity of budgetary and financial resources. Nevertheless, this could hinder the implementation of programming and purchasing of drugs.
The inventory control in health care centers and pharmaceutical supply centers was below that established in the literature, which is 100%, but slightly above the 32% of inventory records for health care centers, 32% in municipal pharmaceutical supply services, and 61% in state pharmaceutical supply services, found in the study by PAHO 26 . Barreto and Guimarães [25] detected a lack of inventory control in 2 municipalities in Bahia, Brazil, and Freitas and Nobre [14] found 100% inventory control in a study in a municipality in Ceará, Brazil, which was manual in health care centers and computerized in pharmaceutical supply centers. However, da Silva [28] found manual inventory control at first and, after the pharmacist arrived, this process became computerized, suggesting pharmaceutical care improvement through operational and managerial measures. Our study reflects the perceived need of support on the part of management for effective inventory control, preferably computerized.
Determining marker drugs is important for collecting indicators. These drugs should be on the National List of Essential Drugs, be among the most used drugs, have low cost per unit, and preferably have international availability [15 ,29 ]. The number of listed drugs may be between 10 and 50 according to the WHO 15 , 20 according to a guideline published by Islam 30 , and between 25 and 50 drugs according to the Rational Pharmaceutical Management Project [31] . In the PNAUM, 58 were deemed key medicines, including herbal medicines 27 .
We found rates higher than those found by Menolli et al. [23] in a study conducted in Londrina (80%), by Moura and Perini 32 , in Minas Gerais (74%), and by Freitas and Nobre 14, in Ceará (70%). According to the Primary Health Care of the SUS, the physical availability of essential drugs verified in the PNAUM only accounted for 52.9% 27 .
The value of the indicator could be even higher than that, since marker drugs, which are part of the CBAF and part of the list of drugs distributed free of charge by the Popular Pharmacy Program (Programa Farmácia Popular) of the Brazilian Ministry of Health 33 , were not in the stock of health care centers of some municipalities we studied. The statement from those in charge of municipal pharmaceutical assistance was that these drugs would be available to users at pharmacies accredited by the government program. This should be further discussed, since, as mentioned previously, not dispensing drugs in health care centers may not prevent but can sometimes hinder access. Therefore, besides the need for regular acquisition of drugs directed to primary pharmaceutical assistance, during the workshop the preparation of protocols with the establishment of criteria for dispensing, or not dispensing, drugs by the Popular Pharmacy Program was discussed, thus aiming to ensure the access needed. Since we found a higher index than the established standard [11] and than several studies in the literature, the provision of important drugs for therapeutic resolution in Primary Health Care in that specific region is deemed satisfactory. Some factors are certainly positively interfering, such as the mechanism for automatic and regular transfer of funds, provision of drugs through the state program, bidding processes for price record (thus decreasing the cost and streamlining their acquisition), and mandatory allocation of federal funds received with municipal counterpart. A limiting factor in the collection of this indicator may be the choice of the drugs determined as markers.
According to the R2 indicator of outcome, the ideal standard is having no expired drugs [11 ,34 ]. In health care centers of the CIR-Ourinhos-SP there were expired drugs available, higher than the 2% found in the state of Rio Grande do Sul [12] and in the study on the evaluation of pharmaceutical assistance in Brazil 26 , whose authors found 0.3% of drugs in this situation. However, Freitas and Nobre 14, in a study in Mombaça (in Ceará state), found 18.75% of expired drugs, but in a secluded space not exposed to users, which is still a risk factor, and which shows potential problems in management. Expired drugs refer to issues related to safety, therapeutic efficacy, and potential problems in the management and infrastructure of pharmaceutical assistance. During the collection of this indicator, we observed drugs whose expiry date could not be identified and more than one manufacturing lot of the product in the same container, available for dispensing, which may cause loss, risk to the users’ health and, once again, may indicate potential problems in management and infrastructure. This indicator was very much discussed during the course of the workshop, since it can be directly related to the training of servants that support the dispensing in pharmacies in the US, and also to the responsibility of pharmacists. Therefore, proper monitoring of the responsible people has been suggested concerning drug fractioning, separation of different lots of drugs in the dispensing area, and training courses for employees of the pharmaceutical assistance field.
The average of prescribed drugs per prescription unit (R3) is an indicator suggested by the WHO. The average of more than two drugs may reflect problems in the prescribing practice 15 . According to Bernardi et al. 12, prescribing fewer drugs per appointments in Primary Health Care, whose main objective is based on the promotion and prevention of health, may represent a rational use of drugs, even with the possible aforementioned variations.
Certainly, there are socioeconomic, cultural, and temporal variables, among others, that may affect these comparisons. In Brazil, Cosendey [34] found an average of 2.11 drugs per prescription in a multicenter study conducted in five Brazilian states. Authors of other studies on different regions of the country found values ranging from 2.0 to 2.4 [12 ,14 ,22 ,23 ,26 ,29 ,,«36 ]. We found the value of 1.9, and therefore it is more favorable than other national results, except for that found by Colombo et al. [37] in Blumenau (in Santa Catarina state), which accounted for an average of 1.8 drugs per prescription.
The indicator of outcome related to the prescribed drugs part of the CBAF of RENAME 2012 (R4) was below the standard level of 70% [11 ,34 ], but higher than that found by Colombo et al. 37 , based on RENAME, which accounted for 57.7%. This indicator shows proper selection, standardization, and adherence of the prescriber to the List of Essential Drugs. The PAHO evaluation of pharmaceutical assistance in Brazil in 2005 showed an average of 78.3% of prescribed drugs present in RENAME, despite the large variation found in the 30 surveyed services, from 48.4 to 97.4%.
In several studies, this indicator was verified based on lists of local drugs with different names: Local List of Essential Drugs ( Lista Local de Medicamentos Essenciais –LLME), by Cunha 35 , finding 92.7% of the prescribed drugs present in it; Municipal List of Essential Medicines (REMUME), by Marcondes 29 , 87%; List of Standardized Drugs ( Lista de Medicamentos Padronizados – LMP), by Santos and Nitrini 36 , 83.4%; List of Essential Drugs, by Naves and Silver 22, 85.3%; List of Primary Drugs ( Relação de Medicamentos Básicos – REMEB), by Bernardi et al. 12, 76%; and finally, the List of Essential Drugs, by Menolli et al. 23, accounting for 73%.
Note that all studies based on standardized lists of local drugs obtained rates higher than our study and the established standard of 70%, which can demonstrate high adherence to standardized drugs, a selection complying with technical criteria or, in fact, standardization based on the prescribers’ demand.
There may be standardization in the survey regarding this indicator, considering RENAME and more specifically drugs of the CBAF, as in the case of studies on Primary Health Care. More actions must be promoted to increase this index, that is, to improve the adherence of prescribers to the standardized list of essential drugs. These actions involve the technical expertise of pharmacists and their capacity to coordinate with prescribers, which was discussed and agreed during the workshop.
In the SUS, prescriptions should be written by generic name, following the Brazilian Common Denomination (BCD) or the International Common Denomination (ICD), according to legislation [38 ,39 ]. In prescriptions researched in health care centers of CIR-Ourinhos-SP, the value found was higher than that of 69.42% found by Cosendey [34] in a research conducted in five Brazilian states; that of 71% found by Marcondes 29 ; that of 30.6%, by Santos and Nitrini 36 ; that of 73.2%, by Naves and Silver 22; that of 64%, by Bernardi et al. 12; and that of 66.5%, by Menolli et al. 23. However, the values were lower compared with those found by Cunha et al. 35 , 84.3%; PAHO 26 , 84.2% (varying from 69.2 to 97.4%); and Freitas and Nobre 14, accounting for 91%.
da Silva [28] reported an increase in prescriptions by generic names from 56.54% in 2003, without the pharmacist, to 75.6% in 2004 after the presence of the pharmacist, suggesting that the influence of the professional’s work would improve the prescription by the nomenclature recommended in the legislation. In a study conducted in Brazil on Access, Use and Promotion of Rational Use of Medicines (PNAUM), 59.6% of physicians reported that they always use BCD or the generic name to prescribe drugs to patients; 31.6% use it sometimes, and 8.8% say they do not use it 27 . Therefore, we observed that although the value found is below the established standard of 100%, encouraged by the existence of the specific legislation, the index was higher than those of many studies in the literature.
In the field research of our study, we perceived that long generic names induce the prescription of brand names, which generally tend to be less extensive. One example was the drug used as a contraceptive, whose generic name is Ethinyl estradiol 0.03 mg + levonorgestrel 0.15 mg, usually prescribed in the US with the brand name Ciclo 21®, which is not always the brand or drug present for dispensing, but whose name is undeniably shorter and easier to remember.
The criteria used by the researchers in the consideration and characterization of the generic nomenclature can also interfere with the collection of this indicator, such as the acceptance or nonacceptance of prescriptions with generic names in the form of abbreviations, such as HCT (hydrochlorothiazide), which is easily identified by the pharmacist and accepted, but noncompliant with the law.
In the workshop we discussed not only compliance with legislation, but also awareness, interaction with prescribers, and possible alternatives to be addressed, such as the aforementioned acronyms of generic names usually practiced. Would that be more reasonable than the brand-named prescription? This issue should be widely discussed by pharmaceutical professionals, since it is not a straightforward decision. It involves legal prerogatives. The population’s ensured access to primary essential drugs is one of the guidelines of the National Drug Policy 24 , and therefore verifying prescribed and available drugs is of extreme importance.
The R6 and R7 indicators – respectively, the percentage of prescribed and available drugs in the US – were lower than the values found by Bernardi et al. [12] in Rio Grande do Sul, which accounted for 88% for prescribed and available drugs, and 84% for prescriptions completely given. In contrast, Marcondes [29] described 60% for prescribed and dispensed drugs. Of three countries that had percentage data of drug dispensing (Bangladesh, Nigeria, and Nepal) in a study conducted by Hogerzeil et al. 40 , two of them had indicators above 80% (Bangladesh and Nepal). A study by Cosendey [34] showed an average of 95% of prescribed and available drugs, ranging from 63.37% in Acre state to 100% in the state of Goiás. Good rates achieved by the CIR-Ourinhos-SP, although a little below the standard of 80% for prescribed and available drugs, especially if compared with the values obtained in Brazil, may demonstrate adequate adherence of prescribers to the list of standardized drugs. This may result from the pharmacists’ work or even from regional characteristics, such as the small population size of most of the studied municipalities, which may facilitate stock management.
It is worth noting our limitation in collecting data of the R6, R7, and R8 outcome indicators, because of the absence of some sort of dispensing identification. For further discussion on access to drug in Primary Health Care, we included the R8 outcome indicator, which refers to the percentage of prescribed and available CBAF drugs, that is, the capacity of health care centers in providing essential drugs at this level of health care. The index obtained for drugs belonging to the CBAF of RENAME, prescribed and available in the health care centers of CIR-Ourinhos-SP, was below the established standard, which is 95% 34 , but higher than 74.94% when considering all prescribed drugs.
The CBAF service is a municipal obligation according to Decree No. 1554/2013 [41] of the Brazilian Ministry of Health; therefore, this indicator should be close to 100%. Failure to comply with this indicator may point to nonadherence of the prescriber to the standardized list of drugs, poor selection of these drugs, and problems in the programming and purchasing of these products.
In the survey on PNAUM, 59.8% of users reported full access to Primary Health Care medicines in the SUS. The Southeast Region, where CIR-Ourinhos-SP is included, had a higher percentage (64.3%), and the lowest was in the Midwest Region (46.3%); 36% of users reported partial access, and less than 5% reported not having access to medicines in the SUS dispensing units 27 .
The thematic workshop was positive, even with the absence of some municipalities. The discussion held by those in the workshop, in addition to enabling the assessment of the local pharmaceutical assistance, provided the development of measures for improvement of the presented indicators.
Our results are unprecedented within the field of regional pharmaceutical assistance, since all primary pharmaceutical assistance of municipalities that compose the CIR-Ourinhos-SP were assessed, through the collection of indicators established and recommended by the literature, and also by preparing, in a democratic and participative manner, an intervention proposal for the practice of the assistance. Thus, we could collect and discuss, in a participative way, indicators of an area of public health that grows and improves, but which still has problems related to structural and organizational aspects and professional training at the municipal level.
We recommend that the intervention proposal be implemented, monitored, and evaluated. Therefore, forming a study group for pharmaceutical assistance of the CIR-Ourinhos-SP would be an important aspect in this process.
We should mention that in 2012 the Brazilian Ministry of Health created the National Qualification Program of Pharmaceutical Assistance within the SUS ( Programa Nacional de Qualificação da Assistência Farmacêutica no âmbito do Sistema Único de Saúde – QUALIFAR-SUS), whose purpose was to contribute to the process of improvement, implementation, and integration of pharmaceutical assistance, both in actions and in health care services [42 ,43 ]. Qualification steps were carried out between 2012 and 2014, and between 2017 and 2018, and 1 municipality of CIR-Ourinhos-SP was on the list of municipalities that qualified in 2014, and 4 in 2018 43 .
REFERENCES
1 Tavares N, Pinheiro R. Assistência Farmacêutica no SUS: avanços e desafios para a efetivação da assistência terapêutica integral. Tempus Actas de Saúde Coletiva. 2014; 8(1): 49–56.
2 Carvalho G. A saúde pública no Brasil. Estud. av. (online). 2013; 27(28): 7–26 (cited 2018 Dec 8). Available from: https://doi.org/10.1590/S0103-40142013000200002.
3 Brasil. Ministério da Saúde. Conselho Nacional de Secretários de Saúde-CONASS. Conselho Nacional de Secretarias Municipais de Saúde-CONASEMS. Orientações Tripartite para o Planejamento Regional Integrado. Brasília, 2018 (cited 2019 Jan 11). Available from: http://www.conasems.org.br/wp-content/uploads/2018/09/PRI-Orientacoes-Tripartite-Terceira-Edicao.pdf. [ Links ]
4 Brasil. Ministério da Saúde. Dispõe sobre a participação da comunidade na gestão do Sistema Único de Saúde (SUS) e sobre as transferências intergovernamentais de recursos financeiros na área da saúde e dá outras providências. Lei Nº 8.080, de 19 de setembro de 1990. Diário Oficial da União, Poder Executivo, 1990 [cited 2012 Jul 3). Available from: http://www.planalto.gov.br/ccivil_03/leis/l8080.htm.
5 Brasil. Agência Nacional de Vigilância Sanitária. Portaria n.º 344, de 12 de maio de 1998. Aprova o Regulamento Técnico sobre substâncias e medicamentos sujeitos a controle especial. Agência Nacional de Vigilância Sanitária. Brasília: Anvisa; 1998 (cited 2014 Mar 13). Available from: http://www.anvisa.gov.br/hotsite/talidomida/legis/Portaria_344_98.pdf.
6 Brasil. Ministério da Saúde. Aprova a Política Nacional de Assistência Farmacêutica. Resolução CNS Nº 338, de 06 de maio de 2004. Diário Oficial da União, Poder Executivo, Brasília, DF, 20 Maio 2004 (cited 2014 Apr 10). Available from: http://www.anvisa.gov.br/sngpc/legis.htm.
7 Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Departamento de Assistência Farmacêutica e Insumos Estratégicos. Relação Nacional de Medicamentos Essenciais: Rename 2012/ Ministério da Saúde, Secretaria de Ciência, Tecnologia e Insumos Estratégicos, Departamento de Assistência Farmacêutica e Insumos Estratégicos. Brasília: Ministério da Saúde; 2012 (cited 2013 Nov 10). Available from: http://portal.saude.gov.br/portal/arquivos/pdf/livro_rename2012.pdf.
8 Brasil. Ministério da Saúde. Aprova as normas de financiamento e execução do Componente Básico da Assistência Farmacêutica. Portaria Nº 1.555, de 30 de julho de 2013. Brasília, 2013 (cited 2013 Nov 10). Available from: http://dtr2001.saude.gov.br/sas/PORTARIAS/Port2007/GM/GM-1555.htm.
9 Donabedian A. La calidad de la atención medica: definición e método de evaluación. Mexico: La Prensa Médica Mexicana; 1984. [ Links ]
10 Castro CG (Coord). Estudos de utilização de medicamentos: noções básicas (online). Rio de Janeiro: Editora FIOCRUZ; 2000 (cited 2014 Feb 10). Available from: http://books.scielo.org https://doi.org/10.7476/9788575412657. [ Links ]
11 Rieck EB. Assistência Farmacêutica na Atenção Básica de Saúde do Estado do Rio Grande do Sul: Análise dos indicadores de avaliação do Plano Estadual de Assistência Farmacêutica Básica (monograph). Porto Alegre: Escola de Saúde Pública da Secretaria da Saúde do Rio Grande do Sul; 2002. [ Links ]
12 De Bernardi CL, Bieberbach EW, Thomé HI. Avaliação da assistência farmacêutica básica nos municípios de abrangência da 17ª Coordenadoria Regional de Saúde do Rio Grande do Sul. Saude Soc. 2006; 15(1): 73–83.
13 Correia AR, Mota DM, Arrais PS, Monteiro MP, Coelho HL. Definição de Indicadores para Avaliação da Assistência Farmacêutica na Rede Pública de Fortaleza (Brasil) baseada em Métodos de Consenso. Lat Am J Pharm. 2009; 28(3): 366–74.
14 Freitas JM, Nobre AC. Avaliação da Assistência Farmacêutica do município de Mombaça (CE). Rev Bras Farm Hosp Serv Saude Sao Paulo. 2011; 2(1): 15–20.
15 World Health Organization (WHO). Indicators for monitoring national drugs policies: a practical anual. Geneva: World Health Organization, 1999 (cited 2012 Aug 18). Available from: http://apps.who.int/medicinedocs/pdf/whozip14e/whozip14e.pdf. [ Links ]
16 Franco MA. Pedagogia da Pesquisa-Ação. Educ Pesqui. 2005; 31(3): 483–502.
17 Tripp D. Pesquisa-ação: uma introdução metodológica. Educ Pesqui. 2005; 31(3): 443– 66.
18 São Paulo (Estado) a. Estado de São Paulo segundo Departamentos de Saúde, 2012 (cited 2012 Jun 30). Available from: http://www.saude.sp.gov.br/ses/institucional/departamentos-regionais-de-saude/regionais-desaude.
19 Brasil. Agência Nacional de Vigilância Sanitária. Formulário de Fitoterápicos da Farmacopeia Brasileira/Agência Nacional de Vigilância Sanitária. Brasília: Anvisa; 2011. p. 126 (cited 2014 Feb 18). Available from: http://www.anvisa.gov.br/hotsite/farmacopeiabrasileira/conteudo/Formulario_de_Fitoterapicos_da_Farmacopeia_Brasileira.pdf. [ Links ]
20 Brasil. Agência Nacional de Vigilância Sanitária. Resolução da Diretoria Colegiada - RDC Nº 95, de 11 de dezembro de 2008/Agência Nacional de Vigilância Sanitária. Brasília: Anvisa; 2008 (cited 2014 Feb 18). available from: http://www.anvisa.gov.br/medicamentos/fitoterapicos/bula_padronizadas_fitoterapico.pdf.
21 Brasil. Ministério da Saúde. Dispõe sobre o controle sanitário do comércio de drogas, medicamentos, insumos farmacêuticos e correlatos, e dá outras providências. Lei Nº 5.991, de 17 de dezembro de 1973. Diário Oficial da União, 1973. Available from: http://www.planalto.gov.br/ccivil_03/Leis/L5991.htm.
22 Naves JO, Silver LD. (Evaluation of pharmaceutical assistance in public primary care in Brasilia, Brazil). Rev Saude Publica. 2005 Apr; 39(2): 223–30. Portuguese.
23 Menolli PV, Ivama AM, Cordoni Júnior L. Caracterización de los servicios farmacéuticos de atención primaria del Sistema Unico de Salud en Londrina, Paraná, Brasil. Rev Panam Salud Publica. 2009 Mar; 25(3): 254–9.
24 Brasil b. Ministério da Saúde. Portaria GM Nº 3.916, de 30 de outubro de 1998. Aprova a Política Nacional de Medicamentos. Diário Oficial (da) República Federativa do Brasil, Poder Executivo, 1998; Nov 10, 1998. s. 1, n.215-E, p. 18. Available from: http://www.cff.org.br/userfiles/file/portarias/3916_gm.pdf.
25 Barreto JL, Guimarães MC. Avaliação da gestão descentralizada da assistência farmacêutica básica em municípios baianos, Brasil. Cad Saude Publica. 2010 Jun; 26(6): 1207–20.
26 OPAS. Avaliação da assistência Farmacêutica no Brasil: estrutura, processo e resultados. Brasília (Brasil): Organização Pan-Americana de Saúde; Organização Mundial de Saúde, Ministério da Saúde, Brasil; 2005 (cited 2012 Jan 13). Available from: http://bvsms.saude.gov.br/bvs/publicacoes/avaliacao_assistencia_farmaceutica_estrutura_resultados.pdf. [ Links ]
27 Brasil. Ministério da Saúde. Secretaria de Ciência, Tecnologia e Insumos Estratégicos. Componente Avaliação dos Serviços de Assistência Farmacêutica Básica: resultados (recurso eletrônico). Brasília, 2017 (cited 2019 Jan 11) Available from: http://bvsms.saude.gov.br/bvs/publicacoes/componente_avaliacao_assistencia_farmaceutica_resultados_1ed.pdf. [ Links ]
28 da Silva Jr DB. Assistência farmacêutica em um município do Estado de São Paulo: diagnóstico e perspectivas (dissertação) Ribeirão Preto: Universidade de São Paulo, Faculdade de Ciências Farmacêuticas de Ribeirão Preto; 2006 (cited 2014 Apr 3). Available from: http://www.teses.usp.br/teses/disponiveis/60/60137/tde-16012007-142838/.
29 Marcondes NS. A assistência farmacêutica básica e o so de medicamentos na zona urbana do município de Ponta Grossa, Paraná: um estudo de caso. Rio de Janeiro: FIOCRUZ/ ENSP; 2002. [ Links ]
30 Islam M. Health Systems Assessment Approach: A How-To Manual. Submitted to the U.S. Agency for International Development in collaboration with Health Systems 20/20, Partners for Health Reformplus, Quality Assurance Project, and Rational Pharmaceutical Management Plus. Arlington, VA: Management Sciences for Health, 2007.
31 RPM (Rational Pharmaceutical Management Project). 1995. Rapid pharmaceutical management assessment: an indicator-based approach. USA: Kumarian Press; 1995 (cited 2012 Aug 10). Available from: http://apps.who.int/medicinedocs/documents/s18650en/s18650en.pdf.
32 Moura CS, Perini E. Evaluation of pharmaceutical assistance in municipalities in the state of Minas Gerais. RBCF. 2009; 45(2): 279– 86.
33 Portal da Saúde. Ministério da Saúde. Brasil. 2014 (cited 2014 Apr 17). Available from: http://portalsaude.saude.gov.br/index.php/o-ministerio/principal/secretarias/sctie/farmacia-popular.
34 Cosendey MA. Análise da implantação do Programa Farmácia Básica: um estudo multicêntrico em cinco Estados do Brasil (Tese)Rio de Janeiro: Escola Nacional de Saúde Pública, Fundação Osvaldo Cruz; 2000.
35 Cunha MC, Zorzatto JR, Castro LL. Avaliação do uso de medicamentos na rede pública municipal de saúde de Campo Grande/MS. RBCF. 2002; 38(2): 215–27. [ Links ]
36 Santos V, Nitrini SM. Indicadores do uso de medicamentos prescritos e de assistência ao paciente de serviços de saúde. Rev Saude Publica. 2004 Dec; 38(6): 819–26.
37 Colombo D, Santa Helena ET, Agostinho AC, Didjurgeit JS. Padrão de prescrição de medicamentos nas unidades de programa de saúde da família de Blumenau. RBCF. 2004; 40(4): 549–58.
38 Brasil. Ministério da Saúde. Dispõe sobre a vigilância sanitária, estabelece o medicamento genérico, dispões sobre a utilização de nomes genéricos em produtos farmacêuticos e dá outras providências. Lei n. º 9787, de 10 de fevereiro de 1999. Diário Oficial da União. Brasília, 1999 (cited 2014 Mar 13). Available from: http://www.suvisa.ba.gov.br/sites/default/files/legislacao/arquivos/2011/07/28/Lei%20Federal%20n%C2%BA%209787%201999%20-%20data%2021.6.pdf.
39 São Paulo (Estado). Resolução SS - 126, de 13- 8-2009. Dispõe sobre a obrigatoriedade de prescrição e dispensação de medicamentos com o nome genérico das substâncias que os compõe. Diário Oficial do Estado, Poder Executivo, Seção I. 2009; 119 (Nº 151).
40 Hogerzeil HV, Bimo, Ross-Degnan D, Laing RO, Ofori-Adjei D, Santoso B, et al. Field tests for rational drug use in twelve developing countries. Lancet. 1993 Dec; 342(8884): 1408– 10.
41 Brasil. Ministério da Saúde. Dispõe sobre as regras de financiamento e execução do Componente Especializado da Assistência Farmacêutica no âmbito do Sistema Único de Saúde (SUS). Portaria Nº 1.554, de 30 de julho de 2013, Brasília, 2013 (cited 2014 Apr 18). Available from: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt1554_30_07_2013.html.
42 Brasil. Ministério da Saúde. Institui o Programa Nacional de Qualificação da Assistência Farmacêutica no âmbito do Sistema Único de Saúde (QUALIFAR- SUS). Portaria Nº 1.214, de 13 de junho de 2012, Brasília, 2012 (cited 2018 Dec 11). Available from: http://bvsms.saude.gov.br/bvs/saudelegis/gm/2012/prt1214_13_06_2012.html.
43 Brasil. Ministério da Saúde. Ministério da Saúde habilita 651 municípios a receberem recursos do Qualifar-SUS. Brasília, 2018 (cited 2018 Dec 11). Available from: http://portalms.saude.gov.br/noticias/sctie/44929-ministerio-da-saude-publica-o-resultadoda-habilitacao-de-municipios-ao-qualifarsus.
Statement of Ethics
This cross-sectional and descriptive study was approved by the Research Ethics Committee of the School of Dentistry at Piracicaba – University of Campinas, under Opinion No. 3/2013 and Certificate of Presentation for Ethical Appreciation ( Certificado de Apresentação para Apreciação Ética – CAAE) No. 7781713.0.0000.5418.
The authors have no conflicts of interest to disclose.














