Introduction
The Relevance of Screening
In 1968, the World Health Organization published guidance on the “Principles and Practice of Screening for Disease” 1 that outlined the following principles of early disease screening:
The condition should be an important health problem.
There should be an accepted treatment for patients with recognized disease.
Facilities for diagnosis and treatment should be available.
There should be a recognizable latent or early symptomatic stage.
There should be a suitable test or examination.
The test should be acceptable to the population.
The natural history of the condition, including development from latent to declared disease, should be adequately understood.
There should be an agreed policy on whom to treat as patients.
The cost of case-finding (including diagnosis and treatment of patients diagnosed) should be economically balanced with possible expenditure on medical care as a whole.
Case-finding should be a continuing process and not a “once and for all” project.
Through a systematic review and a modified Delphi consensus process, Dobrow and colleagues 2 revised and amplified the previous screening principles to also address screening program acceptability and ethics, benefits and harms and screening program quality and performance management 2. These principles were divided into 3 groups: disease/condition specific, test/intervention specific, and program/system specific (Table 1). The higher emphasis toward more program/system principles is related to the type of evidence that could be used to inform screening decisions 2.

*Components of a screening program include recruitment, testing, information access, diagnosis, referral, treatment, follow-up, patient education and support, staff training, and program management and evaluation.
Although most of the above principles apply to lung cancer, and despite its high lethality particularly when diagnosed late, lung cancer screening is still in an early phase compared to other cancer screening programs. Diagnosis of lung cancer at an early stage of disease is strongly associated with improved survival. Regardless of the significant advances in the management of lung cancer in the last decades, the scale of outcome improvement lies far behind what was observed with other cancers, and therefore, achieving early diagnosis will remain crucial to obtaining optimal outcomes 3.
This literature review aims to provide a picture that aggregates the most relevant topics related with lung cancer screening, including its’ benefits and harms, cost-effectiveness, implementation challenges, and potential measures to overcome them that have been subject to an extensive number of publications throughout the years. Additionally, to give an overview of the current situation of lung cancer screening implementation in USA and Europe, as well as the ongoing research focusing on biomarkers for diagnosis that can positively impact its future development. The purpose was to cover in one document the wide variety of aspects and learnings that need to be considered to inform decision makers in case a screening program was to be implemented in Portugal.
Current Evidence for Lung Cancer Screening with Low-Dose Computed Tomography
Over the years, several strategies for implementing lung cancer screening were studied to allow an earlier diagnosis 4 but only in 2011, with the publication of the results of the US National Lung Screening Trial (NLST), funded by the National Cancer Institute, a new paradigm in lung cancer screening was reached 5. The NLST was a randomized trial, to determine whether screening with low-dose computed tomography (LDCT), as compared with chest radiography (CR), would reduce mortality from lung cancer among high-risk asymptomatic persons. Data collection occurred from 2002 through 2009, and focused on a well-defined population aged between 55 and 74 years old, smokers or ex-smokers (that quitted in the last 15 years), with a smoking history equal to or greater than 30 pack-years. A total of 53,454 individuals were randomized in equal proportions (1:1) to perform either LDCT or CR, with an annual frequency, for three consecutive years, and both groups were followed up for further 3 years. The rate of adherence to screening was more than 90%. The relative reduction in mortality from lung cancer with LDCT screening was 20.0% (95% CI: 6.8-26.7; p = 0.004) as compared with CR. The relative reduction in the rate of death from any cause in the LDCT group versus CR was 6.7% (95% CI: 1.2-13.6; p = 0.02). The number needed to screen to prevent one lung cancer death was 320 5. Extended follow-up of the NLST at 12 years showed a number needed to screen (NNS) similar to that of the original analysis 6,7. The NLST demonstrated that in a well-defined high-risk population, potentially eligible for curative surgery, there may be a window of opportunity for early stage lung cancer screening, supported by the observation that most of the LDCT screen-detected cancers were early stage (57.1%) 5.
Nevertheless, of the total number of LDCT and CR screening tests in the three rounds, 24.2% and 6.9%, respectively, were classified as positive. LDCT scans were considered positive if any noncalcified nodule or mass measuring at least 4 mm in any diameter was detected. Across the three rounds, 96.4% of the positive results in the LDCT group and 94.5% of those in the CR group were false-positive results 5. As a consequence, there are safety concerns about potential complications from diagnostic procedures, the financial and infrastructural burden of diagnostic follow-up from false-positive screens, and the undue anxiety and cancer-treatment associated morbidity for asymptomatic indolent tumors 8.
The results previously shown by the NLST were confirmed by a large population-based study conducted in Europe, the Dutch-Belgian lung cancer screening trial (Nederlands-Leuvens Longkanker Screenings Onderzoek (NELSON)). NELSON was a randomized, controlled trial initiated in 2003 that aimed to show a reduction in lung-cancer mortality with volume-based, LDCT lung cancer screening in high-risk male participants 7. A total of 13,195 men (primary analysis) and 2,594 women (subgroup analysis) between the ages of 50 and 74, with a smoking history of >10 cigarettes per day for >30 years or >15 cigarettes per day for >25 years, current or former smokers (who had quit ≤10 years ago) were randomly assigned to undergo LDCT screening at T0 (baseline), year 1, year 3, and year 5.5 or no screening. A minimum follow-up of 10 years until end in 2015 was completed for all participants. Among men, the average adherence to CT screening was 90.0%. The cumulative rate ratio for death from lung cancer at 10 years was 0.76 (95% CI: 0.61-0.94; p = 0.01) in the screening group as compared with the control group, similar to the values at years 8 and 9. Among women, the rate ratio was 0.67 (95% CI: 0.38-1.14) at 10 years of follow-up, with values of 0.41-0.52 in years 7 through 9. In this trial involving high-risk persons, lung-cancer mortality was significantly lower among those who underwent LDCT screening than among those who underwent no screening. There were low rates of follow-up procedures for results suggestive of lung cancer 7. Screening-detected lung cancers were substantially more often diagnosed in stage IA or IB (58.6%) and only 9.4% lung cancers were diagnosed in stage IV 7.
In the NELSON trial, an indeterminate screening test required a repeat CT scan to calculate volume-doubling time before the final screening-test outcome could be defined. Overall, 2.1% (467 of 22,600) of CT scans were test-positive and required further workup by the pulmonologist, leading to 203 screening-detected lung cancers. The overall positive predictive value of a positive screening test was 43.5%. This means that 264 of 22,600 screened participants over all rounds (1.2%) had a false-positive test 7.
The above clinical trials were described in more depth because they led to the recommendations for implementation of lung cancer screening programs in the USA and Europe, respectively. However, additional trials were conducted and the evidence is summarized in a recent Cochrane Systematic review that aimed to determine whether screening for lung cancer using LDCT reduces lung cancer-related mortality and to evaluate the possible harms of LDCT screening 9. A total of 11 clinical trials were included, and besides the two already described above, NLST and NELSON, the other trials were the German Lung Cancer Screening Intervention (LUSI), French DEPISCAN trial, UK Lung Cancer Screening trial (UKLS), US Lung Screening Study (LSS), Italian Detection And screening of early lung cancer by Novel imaging TEchnology trial (DANTE), North American Jewish Hospital Lung Cancer Screening and Early Detection Study, Italian Lung Cancer Screening trial (ITALUNG), Multicentric Italian Lung Detection trial (MILD) and the Danish Lung Cancer Screening Trial (DLCST) 9. This review concluded with moderate certainty evidence that LDCT screening showed a positive impact on lung cancer-related mortality and that the number of invasive and noninvasive interventions is higher in the LDCT screening group compared with the control group, including rates of invasive interventions for non-lung cancer-related disease. However, regarding these invasive interventions, it was considered that no difference in death post-surgery between groups was probable 9. The authors considered that a lack of data regarding the harms of screening remains and recommend that key performance indicators for quality assurance should be part of a screening program to monitor harms such as false positives, complications, recall rates and follow-up 9.
Lung Cancer Screening Recommendations
Following the publication of the NLST, both the United States Preventive Services Task Force (USPSTF) and the Centers for Medicare & Medicaid Services (CMS) recommended lung cancer screening for high-risk persons 10. In 2013, the USPSTF recommended an annual LDCT lung cancer screening in adults aged 55-80 years who have a smoking history of at least 30 pack-years, smokers or ex-smokers for less than 15 years 11-13. The population covered by this recommendation resulted from an analysis performed by the Cancer Intervention and Surveillance Modeling Network (CISNET) that developed several models based on individual data from important studies that estimated the effects of different screening intervals, age at smoking initiation and cessation and smoking history, in which this scenario emerged as the most efficient, after weighing the benefits, risks and the number of screenings that would be necessary to avoid death from lung cancer 11. The number of years needed for screening is not specified, but screening should be discontinued once a person has not smoked for 15 years or develops a health problem that substantially limits the life expectancy or the ability or willingness to have curative lung surgery (grade B recommendation). In March 2021, the USPSTF replaced the initial recommendation for annual screening for lung cancer with LDCT to include adults aged 50-80 years who have a 20-pack-year smoking history and currently smoke or have quit within the past 15 years. The criteria for screening discontinuation remained the same 14. Under the Patient Protection and Affordable Care Act, any procedure that receives a grade B recommendation from the USPSTF has to be covered by private insurers without co-payment 15. The task force recommends using age and smoking history to determine screening eligibility rather than more elaborate risk prediction models because there is insufficient evidence to assess whether risk prediction model-based screening would improve outcomes relative to using the risk factors of age and smoking history for broad implementation in primary care 14.
In 2015, the CMS decided to provide coverage for lung cancer screening in smokers aged 55-77 years with at least a 30-pack-year smoking history and who had quit within the last 15 years 16. In February 2022, CMS expanded beneficiary eligibility for screening for lung cancer with LDCT to closely align with the USPSTF recommendation. CMS is lowering the minimum age for screening from 55 to 50 years reducing the smoking history from at least 30 pack-years to at least 20 pack-years and simplifying screening policy requirements 17.
Other organizations including the American College of Chest Physicians (ACCP) 18, the American Society of Clinical Oncology (ASCO) 19, and the American Thoracic Society (ATS) have incorporated LDCT screening to their recommendations restricting it to the population with criteria that replicate those of the NLST 12,20. The American Cancer Society (ACS) also recommended screening for lung cancer by LDCT with the same criteria as the NLST population, and high priority was given to smoking cessation counseling as screening should not be seen as an alternative to smoking cessation 12,21,22. The American Association for Thoracic Surgery (AATS) recommends screening in a similar population but accepts the extension to risk populations with a lower level of smoking in the presence of comorbidities that increase the risk of developing lung cancer 12,23. The National Comprehensive Cancer Network (NCCN) has recommended screening in populations at risk of developing lung cancer with criteria very similar to the AATS 24. In contrast, the American Academy of Family Physicians does not formally endorse lung cancer screening as they concluded that the evidence is insufficient to recommend for or against screening 25.
The US clinical guidelines reflect uncertainty regarding the stopping ages for LDCT screening 10 with ASCO, ACCP, ACS, and the NCCN guidelines aligned with NLST data at age 74 as the upper age limit 18,19,21,2,24, whereas the USPSTF and the AATS guidelines raise the cutoff to 80 years 10,23,26. Overall, these guidelines offer limited guidance for individualizing lung cancer screening decisions as a function of coexisting illnesses 10.
At the European level, the European Society of Radiology together with the European Respiratory Society (ESR/ERS) recommended lung cancer screening through longitudinal, comprehensive, and quality-assured programs either through clinical trials or in clinical practice in certified multidisciplinary medical centers, with inclusion and exclusion criteria similar to those of the USPSTF. Minimum requirements were defined as the existence of specific norms for the acquisition of LDCT, computerized assistance for nodules evaluation, the management of positive cases in the screening, as well as the implementation of smoking cessation programs 15. It is also strongly recommended to implement a central registry, including a biobank and an image bank, at the European level 15.
To increase the quality, outcome and cost-effectiveness of lung cancer screening the ESR/ERS also recommended the implementation of additional measures namely, the inclusion of risk models, reduction of effective radiation dose, computer-assisted volumetric measurements, and assessment of comorbidities (chronic obstructive pulmonary disease (COPD) and vascular calcification). The specificity of a screening program might also be increased by including nonimaging, noninvasive biomarkers to allow better discrimination between benign versus malignant conditions. Examination of serum and plasma biomarkers shows some evidence supporting the rationale of using these biomarkers for risk stratification of screen-detected lung nodules. However, there are only a few biomarkers which could be implemented immediately 15.
The European Union (EU) has also issued a position statement for lung cancer screening that presents the available evidence and the major issues that need to be addressed to ensure the successful implementation of LDCT lung cancer screening in Europe.
This position statement recommends the following actions:
A risk stratification approach should be used for future lung cancer LDCT programs
Individuals who enter screening programmes should be provided with information on the benefits and harms of screening, and smoking cessation programs should be offered to all current smokers
Management of detected solid nodules should use semi-automatically measured volume and volume-doubling time
National quality assurance boards should be set up to oversee technical standards
A lung nodule management pathway should be established and incorporated into clinical practice with a tailored screening approach
Noncalcified baseline lung nodules greater than 300 mm3, and new lung nodules greater than 200 mm3, should be managed in multidisciplinary teams to ensure that patients receive the most appropriate treatment 26.
The EU position considers that setting national central registries for screening participants would ensure that inclusion criteria are met. The institutions providing a lung cancer screening service should be registered, have access to information from previous screens, use certified nodule evaluation software, and deliver screening results and recommendations to a central registry. Institutions participating in screening programmes require multidisciplinary teams representing all relevant specialties to enhance the discussion of suspicious screening results. One best practice example from the Dutch breast cancer screening programme that the lung cancer community should consider is the implementation of CT central reading centers 26. In a multidisciplinary context, monitoring the quality-of-care is critical for the success of lung cancer screening. The comparison of practices and results between the various screening centers is necessary to ensure high-quality standards at the national level 27.
On November 29th, 2022, the Council of the European Union emitted a recommendation on strengthening prevention through early detection (2022/0290) that replaced the previous Council recommendation from 2003 (2003/878/EC), urging Member States to explore the feasibility and effectiveness of lung cancer screening programs (among other types of cancer) targeting high-risk individuals, for instance through implementation studies. Among other things, these studies should reinforce research on how to reach and invite the target population of heavy smokers or ex-smokers considering the lack of systematic data on smoking behavior 28.
Lung Cancer Screening Criteria
The findings of both NLST and NELSON need to be translated into advice on “who to screen” (high-risk group), “how often” (intervals between rounds), and “for how long” (age cut-off) 29. Lung cancer screening is likely the first large-scale cancer screening programme that relies on additional risk factors to select the population at risk, mostly tobacco consumption. Both NELSON and NLST selected participants based on age and smoking but other trials and pilot programmes have selected based on multivariable risk prediction models 30. The use of individual risk is superior to selection criteria based on age and pack-years alone 31, and therefore lung cancer screening could become the first major targeted cancer screening programme 30.
A significant challenge in risk-based screening is how to incorporate comorbid conditions into estimates of the benefits and harms of lung cancer screening. For example, patients with COPD are at increased risk of death from lung cancer and have the most to gain from screening, but the presence of a chronic lung disease also limits life expectancy and increases the risk of complications from the subsequent diagnostic and therapeutic procedures 10. In this specific comorbidity, available evidence supports the inclusion of patients with mild to moderate COPD (GOLD grade 1 and 2) in LCDT lung cancer screening as it does not lead to over-diagnosis and may result in a high percentage of diagnosis in early curable stages with significantly improved mortality 32. However, the benefits to those screened with advanced COPD (GOLD grade 3 and 4) remain controversial since findings from a NLST sub-study show that the rates of respiratory deaths are higher than lung cancer deaths in that population 10,33.
Lung cancer screening can be an inefficient process even in high-risk groups such as cigarette smokers, as only a small proportion of individuals will be diagnosed with lung cancer 34. Inviting asymptomatic individuals for screening and implementing a large-scale screening program should be considered only when the benefits outweigh the harms 35. Since lung cancer is a disease of aging and the frailty burden increases with age, evaluating frailty in the lung cancer screening setting may help identify subgroups of patients less likely to benefit from screening 10. Screening should not be performed in individuals at elevated risk who do not have the physical capacity required to receive appropriate treatment. Performance status and lung function should be used to determine on an individual basis when to stop lung cancer screening 36. The severity of comorbidities among elderly patients may influence LDCT referrals for lung cancer screening as a potential benefit of screening is minimal for people with a major health problem that substantially limits life expectancy or the ability to receive curative lung surgery 37. However, extremely elderly patients who are well may also benefit from continued screening beyond the age 79 years on an individual basis 36. The magnitude of the benefits expected must be weighed against the risks and costs because resources are not unlimited and society should decide how to implement a responsible screening programme based on evidence-based data 34.
Improving Screening Criteria through Risk Models
The clear definition of a target population for lung cancer screening is important to maximize its efficiency. The ability to predict which individuals are at high risk for developing lung cancer using age and smoking history criteria alone is limited 38. Analysis of the Surveillance, Epidemiology and End Results (SEER) database has shown that only 26.7% of patients with lung cancer in the USA would have been eligible for LDCT screening by NLST criteria 3,39. Adding additional risk factors may improve risk prediction and screening performance 38. Thus, the use of composite risk prediction tools may better identify the high-risk population and increase the proportion of lung cancers that may be detected by screening 3. Several multivariable risk prediction models have been published and although the EU position statement does not recommend any specific risk prediction model, it mentions that there are two of them, the modified Liverpool Lung Project (LLPv2) and the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCOm2012) models that could be enough if screening was to be implemented immediately 26.
The Liverpool Lung Project (LLP) aimed to provide a model that would estimate the absolute risk of lung cancer for a given individual, which could be used to help identify those most likely to benefit from screening 40. The variables included in the final model needed to be readily available to primary care clinicians, so that it could be applied in the primary care setting to facilitate the referral of high-risk individuals. As well as accounting for the three most important risk factors for lung cancer: age, sex and smoking, the LLP risk model incorporated other important disease risks factors such as the family history of lung cancer, occupational asbestos exposure, prior diagnosis of pneumonia and prior diagnosis of a malignant tumor other than lung cancer 40. Significantly increased risks in the multivariate analysis were observed for family history of lung cancer (particularly in those with a relative aged under 60 at diagnosis of lung cancer) (p = 0.01), prior diagnosis of pneumonia (p = 0.002), prior diagnosis of cancer other than lung (p = 0.005), occupational exposure to asbestos (p < 0.001), and duration of smoking (p < 0.001) 40. The results of the LLP risk model suggest that it could predict approximately two-thirds of lung cancers within 5 years, screening only 30% of the population. However, Caucasians represented approximately 99% of the population covered 40.
The LLPv2 risk model cutoff of 5% over 5 years has also been used in the Liverpool Healthy Lung project 41, which has accommodated the risk model within primary care practice and produced risk assessments that are useful in clinical decision-making. Models such as these provide a systematic way of assessing lung cancer risk, taking into account a range of factors, including smoking duration, previous respiratory disease, family history of lung cancer, age, previous history of malignancy and asbestos exposure. Risk stratification in primary care is a key priority 42.
The PLCO models aimed to produce improved lung cancer risk prediction models by incorporating a wider range of lung cancer risk factors which included age (55-74 years), socioeconomic status (estimated by education), race/ethnicity, sex, family history of lung cancer, body mass index (BMI), history of COPD, history of chest x-ray in the 3 years before baseline, smoking history (smoking status, smoking intensity, smoking duration, and smoking quit time) 43. The PLCOm2012 model was a modification of the previous PLCO model for the sub-cohort of ever-smokers to ensure applicability to the NLST data. As compared with NLST criteria, PLCOm2012 criteria had improved sensitivity (83.0% vs. 71.1%, p < 0.001) and positive predictive value (4.0% vs. 3.4%, p = 0.01), without loss of specificity (62.9% and 62.7%, respectively; p = 0.54) and 41.3% fewer lung cancers were missed. As the NLST screening effect on mortality reduction did not vary according to PLCOm2012 risk (p = 0.61 for interaction) the use of the PLCOM2012 to select individuals for lung cancer screening programs should translate into more efficient selection for screening (a higher number of cancers detected per number of individuals screened), greater cost-effectiveness, and additional lives saved from LDCT screening 44. More recently, the PLCOm2012 model (at a threshold of 1.51% probability of lung cancer over 6 years) demonstrated to perform better than USPSTF criteria (sensitivity 80.1% vs. 71.2%, specificity 66.2% vs. 62.7%, and positive predictive value 4.2% vs. 3.4%) 38,45.
Models such as LLP and PLCO might be used to identify risk for patients who would otherwise not receive lung cancer screening. These individual risk assessments can be used by patients and providers to assess if one is at substantial risk for developing lung cancer 46. There is a common misconception that only heavy smokers develop lung cancer, however, 25% of all lung cancer cases in the USA occur in never smokers, particularly women. Therefore, the development of lung cancer risk prediction models that incorporate individual variables other than tobacco smoking is useful 46. Further to smoking, the next strongest variable for risk was a first-degree relative developing lung cancer before the age of 60 years. Online risk calculators can facilitate the cumulative effect of multiple variables 46.
Available from the LLP investigators is a web-based tool that facilitates the calculation of individual risk, which can be obtained free of charge 46,47. MyLungRisk is an easy on-line lung cancer risk prediction questionnaire, which can calculate the individual risk of developing lung cancer over the next 5 years, based on the LLP risk model. It has been developed for use by individuals aged 50-79 years and is based on age, gender, smoking duration, family history of lung cancer, previous history of pneumonia, previous diagnosis of cancer and exposure to asbestos 47. The questionnaire can be completed by nonsmokers, former smokers, and current smokers (https://liverpoollungproject.org.uk/risk-model/). The PLCO risk models can be obtained as Excel spreadsheet calculators 46,48.
Nevertheless, the use of the LLP and PLCO mathematical models to predict the risk of developing lung cancer in populations other than the ones considered has limitations. One of those is the concerns with the model’s ability to generalize and extrapolate to broader and more diverse cohorts. As an example, the LLP covered approximately 99% Caucasians, and accuracy might be reduced when applied to other ethnicities. Similarly, the PLCO model included individuals aged 55-74 years and on average with a higher socioeconomic status, which if applied to the general population with wider ranges of age, education, and socioeconomic status might result in limited benefits. Another is the absence of potential predictors such as second-hand smoke, radon, and occupational exposure that were excluded from these models and could alter the predictive accuracy 46.
Determining eligibility for lung cancer screening using more complex risk prediction models may represent an implementation barrier, and there is currently insufficient evidence to assess whether risk prediction model-based screening would improve outcomes relative to simply using the risk factors of age and smoking history 14. The International Lung Screening Trial (ILST), a prospective cohort study that compares the accuracy of the PLCOm2012 model against the 2013 USPSTF criteria for detecting lung cancer, may provide some evidence regarding this issue 49. The ILST also aims to evaluate nodule management efficiency using the PanCan nodule probability calculator-based protocol versus Lung-RADS. The PLCOm2012 (Prostate, Lung, Colorectal and Ovarian) Cancer Screening Trial 6-year and PanCan (Pan-Canadian Early Detection of Lung Cancer) nodule malignancy risk models are two of the better validated risk prediction models for screening selection and nodule management, respectively. Combined use of these models for participant selection and nodule management could significantly improve screening efficiency. ILST recruited 2,000 participants who met USPSTF and/or PLCOm2012 risk ≥1.51%/6-year selection criteria. Participants will undergo baseline and 2-year LDCT screening. Baseline nodules are managed according to the PanCan probability score. Participants will be followed up for a minimum of 5 years. The study completion date is estimated for Dec 2023 49,50.
Screening Frequency and Duration
Annual screening for lung cancer has been proposed as a method to improve patient outcomes, supported by the results from several screening trials that showed that LDCT screening in high-risk individuals increased detection of early stage disease and reduced lung cancer mortality 34. For a screening program to be effective, participants must return for annual follow-up if they continue to meet eligibility criteria and comply with follow-up testing in case of a positive finding. The NLST reported 95% compliance over 3 years of annual screening; however, screening compliance can be compromised if individuals have to pay for their CT scan 38.
Reduced frequency screening, for every 2 or 3 years appears to lower both the number of scans performed and the expected lung cancer mortality reduction to one-half or one-third compared to annual screening. The number of radiation-induced deaths also decreases by one-half or one-third 38. The modeling efforts and a judgment about the trade-off of mortality reduction and harm led the USPSTF to recommend an annual screening interval up until age 80 years, assuming one remains healthy enough to benefit from treatment for a screen-detected cancer 38.
Cost-effectiveness is also an important consideration affected by the frequency and duration of lung cancer screening. Evidence from a detailed model suggested that annual screening was more cost-effective than longer screening intervals 31,38.
To enable further depth on these issues, EU funded the 4-IN-THE-LUNG-RUN project that is a randomized controlled trial amongst 24,000 individuals evaluating whether it is safe to have risk-based less intensive screening intervals after a negative baseline CT. It is a multicenter implementation trial in 6 different healthcare settings (Netherlands, United Kingdom, Germany, Spain, Italy, France) with an additional focus on individual optimal recruitment and smoking cessation strategies, co-morbidity reducing strategies (including other markers on CT imaging, as COPD and the use of a calcium score for CVD), and biomarkers. Cost impact and cost-effectiveness analyses using a natural history model will steer implementation. This proposal will form the evidence base for risk-based lung cancer screening with huge benefits for the EU, on health outcomes, cost savings, and innovation in the long run. Estimate completion December 2024 51.
Benefits and Harms
It has been demonstrated that any shift toward a lower stage at diagnosis brings substantial benefit in lung cancer patients’ survival 52. The advances in lung cancer screening using LDCT demonstrate that it is possible to detect lung cancer at an early stage and thereby reduce morbidity and mortality 10. Existing data indicate that the majority (85%) of lung cancers found in new incident nodules during lung cancer screening are detected at stage I 26,53.
However, it is important to highlight that a high frequency of early stage cancers is only beneficial if simultaneously the frequency of late stage cancer is reduced, implying a reduction in mortality. If this is not the case, a high frequency of early stage cancer may be due to overdiagnosis, and this can be potentially harmful because it may imply unnecessary investigation and treatment 54.
Additionally, lung cancer screening can be a critical moment to offer and convince high-risk smokers to enter a smoking cessation program. Although it is not clear if the participation in a screening programme influences the quit rate, the existing evidence suggests that this population is motivated to cease smoking 30,55. Nevertheless, the insufficient research in this area should not limit the provision of smoking-cessation interventions for these smokers 30,56.
Despite the associated benefits, the implementation of lung cancer screening by LDCT has some problems, including the high false-positive rate and overdiagnosis of situations which require further unnecessary interventions, sometimes invasive 10,57 with the inherent emotional disturbance, the costs associated to screening, the possible long-term cumulative risks of exposure to radiation and the fact that smokers undergoing screening may feel motivated to continue smoking following a negative CT scan 4,11,58-61. False positives are abnormalities that after further investigation turn out not to be diseases 62. In the 2022 Cochrane review of LDCT clinical trials, the combined baseline results revealed that 21% of trial screens had a false-positive result, with a range from 1% to 46%. The false-positive rate of LDCT was lower in trials that used volumetric analysis alone, which ranged from 1% to 5%, compared with diameter criteria alone which ranged from 18% to 26%. The trials that used both diameter and volumetric criteria had false-positive rates between 8% and 46% 9. The differences in the screening methodology, introduced by the NELSON trial that developed a noninvasive protocol based on volume measurement and growth rate, instead of diameter only, resulted in close to 10-fold reduction of the false-positive rate compared to the NLST 29.
Overdiagnosis is defined as the detection of a cancer that would not otherwise have become clinically apparent and is often an intrinsic feature of screening 63. Indolent or slowly progressing cancers likely cause little harm if left untreated, but harm increases with treatment due to the risks associated with lung cancer resection or other interventions 64. In the NLST, there was an 18.5% (95% CI: 5.4-30.6%) probability that any lung cancer screening with LDCT was an overdiagnosis 63. In the Cochrane review, the estimated overdiagnosis at 10 or more years showed that combined risk was 18%, but the 95% CI was wide, with a lower limit of the 95% CI just below 0 and an upper limit of 36%, suggesting possibly no difference between the groups 9. The psychological harms associated with overdiagnosis can be reduced by providing information about LDCT screening in a language that is understood by those who are screened, including details about atypical findings, with accurate information about the probability of cancer, especially where findings are potentially benign 26.
In the Cochrane systematic review, there was some evidence to suggest there were no long-term psychological harms from screening, with some people in the CT screening group feeling less anxious compared to the control groups who were not offered screening 9. In terms of psychosocial consequences, the evidence was of low certainty due to inconsistencies in outcome measures, sample groups, and timing of assessments. Overall the limited evidence available did not suggest any long-term adverse impact on psychosocial well-being or HRQoL with LDCT screening 9.
The radiation risk is likely to be overestimated and will decrease in the future with the ultra low-dose CT technology 26. From the age of 50, which is often considered a starting point for LDCT, radiation-induced cancer risks decrease significantly with increasing age of the participant 30. In an analysis of the cumulative radiation exposure and lifetime attributable risk of cancer incidence associated with lung cancer screening using annual LDCT, it was estimated that cumulative radiation dose over a period of 10 years is 13 mSV in women and 9.3 mSV in men. Thus, one major cancer would be caused by radiation for every 108 patients with the diagnosis of lung cancer. Radiation exposure and cancer risk from LDCT screening for lung cancer, even if non-negligible, can be considered acceptable in light of the substantial mortality reduction associated with screening 65.
Minimizing harm is essential to maximize the clinical effectiveness of the intervention. It must be ensured that only patients with a high risk of developing lung cancer are screened, screening radiation dose should be reduced to a minimum, and the effective management of atypical findings, including nodules, suspected lung cancers, and incidental findings 26.
Additionally, an important implication of lung cancer screening by LDCT is its impact on workforce capacity of radiologists and thoracic surgeons in the management of detected cases, and for successful screening implementation capacity planning is essential 30. Following the release of USPTF recommendations, an analysis done in the USA, estimated that scaling up lung cancer screening would increase imaging procedures by an average of 4% across Health Service Areas 66. Optimization of resources could be achievable with reduction of lung cancer screening intensity, setting intervals of more than 1 year in cases with minor CT findings in which the risk is low, and who represent a large proportion of the screening population 67. Artificial intelligence algorithms will be necessary to relief pressure on radiologists and radiology services and will play a role in different tasks of the screening process. For now their most appropriate function is that of a second reader in order to increase sensitivity for nodule detection, but AI can also have a role in automatically categorizing screen detected pulmonary nodules 67.
A study conducted in Canada reported that implementing LDCT lung cancer screening could increase the number of operable lung cancer cases per thoracic surgeon by 19.8% in 2030 68. A projection done regarding the USPSTF policy, assuming an adherence rate of 50%, estimated that 37% more lung cancer surgeries would be required in 2015-2040 compared to no screening. The full-scale implementation of lung cancer screening causes a major increase in surgical demand, with a peak within the first 5 years, and strongly influenced by adherence 69.
Nodule Management
Small nodules are extremely common but the vast majority of these nodules are benign. Fewer false-positive nodules reduce the need for further work-up and the risk of complications, especially from invasive diagnostic examinations including surgery 15. The NLST defined any noncalcified nodule measuring at least 4 mm diameter as a positive screening result, suspicious for lung cancer 5. As a consequence, the number of false-positive scans was high: 27% of scans in the first two screening rounds, of which 96% were false-positive. According to the NLST nodule management algorithm, these suspicious nodules needed further work-up, either a follow-up LDCT for nodules of 4-10 mm or a referral to a pulmonologist for nodules >10 mm in diameter 5,15.
In the NELSON trial, tumor volume and volume doubling times were effectively used to reduce the number of positive scans 34. For a positive screening, the NELSON trial used a threshold of 9.8 mm diameter (500 mm3 volume) for any noncalcified nodule with a solid component but also established an indeterminate group of nodules measuring 4.6-9.8 mm in diameter (50-500 mm3 volume) or a nonsolid nodule with a diameter >8 mm that required earlier follow-up than the yearly screening interval 15,70. These nodules were only considered a positive screening result if an increase in volume of at least 25% was found 70. Through this approach, the number of scans with positive screening results was reduced from 27% in the NLST to 2.7% in the NELSON, and the false-positives could be reduced substantially from >95% in the NLST to approximately 50% in the NELSON trial 15.
An analysis was conducted to the participants of the NELSON trial that had solid nodules registered as new or <15 mm3 (study detection limit) at previous screens and received additional screening after initial detection. More than half of new low-risk and intermediate-risk solid nodules in LDCT lung cancer screening resolve. At first LDCT screening after initial detection, volume doubling time (VDT) (AUC: 0.913) and volume (AUC: 0.875) had high discriminatory power. The combination of VDT and the previously established ≥200 mm3 high-risk cut-off (AUC: 0.939) outperformed volume alone but was not significantly better than VDT alone (p = 0.0535). Using a VDT cut-off ≤590 days, together with the ≥200 mm3 high-risk cut-off, and classifying nodules as positive when at least one criterion was fulfilled, provided 100% sensitivity and 84% specificity and 27% positive predictive value for lung cancer 71.
The European position statement on lung cancer adopted a ≥200 mm3 cut-off for high-risk new solid nodules from the NELSON trial’s results 26,71. Nodule risk stratification is based on a nodule’s lung cancer probability, with only high-risk nodules (commonly >15% lung cancer probability) warranting immediate referral of a participant to a specialist, whereas low-risk (<27 mm3 in volume, <1% lung cancer probability) and intermediate-risk nodules (27-207 mm3 in volume, 3% lung cancer probability) receive additional screening LDCT scans 71.
The EU recommendation for the future management of solid nodules detected with CT screening is that semiautomatically derived volume and volume-doubling time should be used in preference to diameter measurements, which should only be used where volumetry is not technically possible 26. A study using the NLST database showed that compared to radiologist reading, the computer-aided diagnosis (CAD) image analysis method significantly improved diagnostic accuracy for lung nodules detected by LDCT, from 70% to 91% (p = 0.018) 72. By helping radiologists distinguish benign lesions from malignant ones, CAD has the potential to reduce the morbidity associated with LDCT screening, including radiation exposure, overdiagnosis of incidental findings, and anxiety, as well as to reduce unnecessary testing and the financial costs of lung cancer screening 72.
One of the best known risk models for nodule management is the 2013 McWilliams model (also known as the Vancouver, PanCan, or Brock model) 73, which assigns weighting factors to multiple variables, including specific morphologic nodule features observed by a radiologist, to determine the likelihood of cancer in screening-detected nodules. It has achieved impressive accuracy in independent data sets, including cases from the National Lung Screening Trial (NLST) and Danish screening trials, with superior ability to predict malignancy compared with the American College of Radiology Lung CT Screening Reporting and Data System, or Lung-RADS, and National Comprehensive Cancer Network guidelines 74. Additional evidence on the efficiency of nodule management is expected from the International Lung Screening Trial (ILST) described earlier in this paper 49. New developments, such as deep machine learning, will assist in the automation of pulmonary nodule management in lung cancer screening 26,75.
Evidence on Cost-Effectiveness
Implementing a lung cancer screening program as a high-quality preventive health service is complex and poses logistical and financial challenges because many variables can contribute to the magnitude of the benefit in terms of screening-associated mortality. As with other screenings, it is essential to provide a safe, cost-effective and accessible service to individuals at-risk and properly informed 76.
Considering the limited health resources, the cost-effectiveness of lung cancer screening is an important societal consideration 38. A cost-effectiveness study of the NLST showed that LDCT screening costs an additional USD 1,631 per patient or USD 81,000 per quality-adjusted life year gained in comparison to no screening which is below the considered USD 100,000 threshold 77. The cost component of LDCT screening includes not only the screening examination itself but also the diagnostic follow-up and treatment 5. A study that estimated the costs and benefits of annual lung cancer screening offered as a commercial insurance benefit in the high-risk US population ages 50-64 found that the cost per life-year saved would be below USD 19,000, an amount that compares favorably with screening for cervical (USD 50,000-75,000), breast (USD 31,000-52,000), and colorectal (USD 19,000-29,000) cancers 78.
In a pilot study conducted in the UK, the UK Lung Cancer Screening (UKLS), the cost component associated was also analyzed, and an incremental cost-effectiveness ratio (ICER) of GBP 8,466/quality adjusted life-year was estimated for a single LDCT scan compared to current practice 79. In this study, a population-based questionnaire was used to identify individuals at high risk, and the management of nodules detected by CT was based on a pre-specified protocol. Of the 2,028 randomized in the CT arm, 42 (2.1%) patients had confirmed lung cancer and of these, 85.7% (36 patients) had stage I or II lung cancer which in more than 90% of cases could receive potentially curative treatment. Economic evaluation suggests that the intervention will be cost-effective, but this analysis needs confirmation using observed mortality reduction data. The ICER will be less favorable if there is substantial overdiagnosis but better if the smoking cessation rate is improved 79,80. A study in the USA found that repeat annual lung cancer screening in a high risk cohort of adults aged 50-64 is highly cost-effective and offering smoking cessation interventions with the annual screening program improved the cost-effectiveness of lung cancer screening between 20% and 45% by increasing the number of quality adjusted life-years saved 81. Other studies in the UK 82 and Switzerland 83 have also found that lung cancer screening may be cost-effective.
A recent systematic review conducted for lung cancer screening with LDCT showed that the large majority of the studies found this methodology to be cost-effective 84. Across the studies, cost-effectiveness was optimal in those aged 55-75 years and smoking history of at least 20 pack-years. Biennial screening was often more cost-effective than annual screening. A smoking cessation intervention alongside screening improved cost-effectiveness, but it was not clear which type of intervention was optimal. Risk prediction models using more parameters to target participants for screening did not have more benefits than those using age and smoking alone, and cost-effectiveness was equivalent. Cost-effectiveness was sensitive to cost and specificity of LDCT, and disutility associated with screening due to frequent indeterminate findings. The three most cost-effective scenarios used a NELSON-like nodule approach 84.
The decision to implement screening programs should be based on information on resources use and costs that are a substantial component of any lung cancer screening program and should be considered in cost-effectiveness analysis 80. In this sense, an analysis was performed of the costs and resources used in the screening and treatment of lung cancer in the initial years of the Pan-Canadian Early Detection of Lung Cancer Study from the perspective of the public payer. The analysis concluded that the average cost of screening an individual at high risk of developing lung cancer using LDCT and the average cost of initial treatment with curative intent were lower than the average cost of treating an advanced-stage lung cancer that infrequently results in cure 80. As new targeted and immunotherapy agents emerge with increasing costs of treatment for advanced stages of disease, and their use becomes wider, a screening program could potentially generate savings while improving health outcomes 80.
A screening program combined with smoking cessation campaigns can improve the cost-effectiveness of the program 76. In fact, an increase in the number of people quitting smoking as a result of the introduction of lung cancer screening significantly improves the cost-effectiveness of the procedure 85. The incorporation of the coronary artery calcification score and emphysema assessment on LDCT imaging might also enhance the cost-effectiveness and attractiveness of LDCT lung cancer screening 86. COPD and emphysema are the strongest lung cancer risk predictors and, together with cardiovascular disease, all three imaging biomarkers have substantial effects on morbidity but also have independent effects on overall mortality 26,87.
Implementation of Lung Cancer Screening Interventions
Screening Adoption in the USA
In the USA, soon after the publication of the NLST results, lung cancer screening received broad support from professional organizations and insurers but the uptake has been very limited 3,88. Participation rates were 3.3% of the eligible population in 2015 and more recently estimated to be 14% in 2018 although only 4% in the uninsured 89. In the first one million screened from 2015 to 2019 in the American College of Radiology (ACR) registry, adherence to annual screening was only 22.3% 90. The lack of a coordinated national approach can contribute to this situation 3. Another study indicated that lung cancer screening programs are suboptimally distributed and this partly contributes to the slow adoption rates among the eligible population 91. Therefore, it is important to evaluate the detailed geospatial distribution of lung cancer screening facilities, and how it evolves over time to identify whether the screening needs of at-risk populations in a particular region are being met 91. Increased societal and physicians awareness and decreased stigma related to tobacco will improve lung cancer screening rates, but it is also very important to improved access and referrals to high-quality lung cancer screening programs 91. Identification of patients at risk of lung cancer who require further investigation is an important responsibility for general practitioners (GPs) that need to maintain an appropriate level of suspicion and readiness to investigate high-risk patients to achieve early diagnosis 3,42.
Successful guideline implementation accelerates the translation of research advances into clinical practice, but the uptake of cancer screening guidelines is a slow process that can be challenging and complex for practitioners in clinical or community-based settings 88. Although the technological capabilities and the knowledge to significantly reduce the burden of lung cancer exist, a greater progress will only happen through a cultural change that leverages the value of early detection of cancer for all the health stakeholders 88.
In what relates to patients, inconvenience due to time constraints and scheduling conflicts, health-care system distrust and perceived smoking-related stigma may lead to low levels of patient engagement with medical care and decreased cancer screening participation 92. The stigma associated with feelings of shame and self-blame can influence timing for seeking medical help that is significantly associated with poorer quality of life, more depressive symptoms and lower levels of adherence to medical care. Understanding individual health beliefs about screening among long-term smokers will help future efforts to facilitate shared decision making about lung cancer screening participation, which is a requirement of CMS coverage. It is essential that screening-eligible patients receive education and counseling regarding the procedure 92.
If the individuals believe they are at risk of cancer, that screening will reduce the consequences through early detection, that the benefits of participating in a screening program outweigh the perceived barriers, and that they can accomplish the tasks necessary for the screening process they will be more likely to participate 92. The involvement of screening-eligible individuals in the development of communication materials 92 and the adoption of non-confrontational communication strategies that normalize the offer and reduce blame could improve engagement of more dependent smokers 93.
Situation in Europe
Europe has proven more cautious toward initiation of lung cancer screening programmes with countries adopting different attitudes 30,94. Croatia was the first and only country to implement a national lung cancer screening with LDCT in October 2020 restricted to those aged 50-70 years, active smokers with a smoking history of 30 pack-years, or ex-smokers who quit smoking within the last 15 years 30,95. Based on the results of the NELSON trial that showed lung cancer screening with LDCT works, Croatian health authorities considered that there was no point in running a pilot. The goals of the programme include saving more than 500 lives per year through a 20% reduction in lung cancer mortality within the next 5-10 years and raise 5 year survival rates from 10% to 15%. Family doctors are central to the screening organization and responsible for patient identification during routine visits and referral. This task is facilitated by the existing databases that include patient smoking habits related to other conditions such as COPD and heart disease 95.
In England, the approach was different and they introduced pilot lung cancer screening programmes in 2019, with a 3-phase roll-out across the National Health Service (NHS) that will provide early insights on potential challenges and pitfalls, allowing an ongoing programme evolution 95. The pilot programmes are linked to the NHS Long Term Plan, launched in 2019 that aims to reform the NHS in a 10-year timeframe. As regards cancer the plan has the ambition that 55,000 more people would survive their cancer, and to secure that aspiration is critical to increase the number of early diagnosis. The first 10 lung cancer screening pilots were situated in areas having the highest lung cancer mortality in England. Ever smoker individuals aged 55-75 years are being invited for the lung health check, identified through general practitioner records, and with local advertising also to encourage eligible people to show-up for the check. The check uses two separate risk calculators, the LLP and the PLCO. Only those considered at high risk are referred on for a LDCT scan. Smoking cessation is considered a core part of the lung health check, with smoking cessation services offered to all who want to quit 95.
The majority of the projects use mobile CT scanners that are placed in easy to reach locations, such as supermarket car parks. This change originated from the evidence generated by the Manchester Lung Health Check pilot conducted in deprived areas, which showed that providing local LDCT scanning services to high-risk individuals (6-year risk ≥1.51%, PLCOm2012 calculator) close to where people live increased the lung cancer detection rate with one lung cancer detected for every 33 people undergoing an LDCT scan 95,96.
In 2016, the French Haute Autorité de Santé decided that they would not introduce an organized lung cancer screening due to the high number of false-positives. However, following the results of the NELSON trial, the scenario changed because the trial not only showed reductions in mortality but also that screening strategies using nodule volume doubling time had few false positives 95. Currently in France, the exploratory CASCADE lung cancer screening pilot is being developed focusing only on females aged 50-74 years, smokers with a smoking history of 25 pack-years or ex-smokers who quit within the previous 15 years. This pilot aims to explore the gender differences revealed in the NELSON study, which showed that while among men screening led to a 24% reduction in lung cancer mortality, among women the reduction in mortality was 33% 95. A shortage of expert thoracic radiologists to read LDCT scans is predicted to be a major problem in France if lung cancer screening becomes widespread. Investigators are planning to assess whether it is possible to take general radiologists and train them to perform low-dose CT. As the pilot develops, the team plans to introduce smoking cessation and combine lung cancer screening with other forms of LDCT appropriate for women of the same age range, including screening for breast cancer and osteoporosis 95.
German sickness funds are considering reimbursement of lung cancer screening as of 2022, but details of inclusion criteria and interval are lacking 30. Other countries, such as Spain, have no pilot initiatives planned, with lung cancer screening available only on a private basis or as part of a European clinical trial 94.
There is growing consensus that further randomized trials for the confirmation of the NLST and the NELSON trials to address the issue of effectiveness of low dose CT-scan are worthless and that the efforts should now be directed at implementation in Europe in a progressive but irreversible manner in all EU countries 30. Harmonization of the screening program across the EU will happen as the evidence accumulates 30.
Maximization of the screening participation rate is a very important issue that needs to be addressed to potentiate programme success. Informed uptake, participation, and adherence to successive screening rounds, determine the overall impact of the intervention by ensuring that the maximum number of people at high risk of disease are screened regularly to improve the chance of benefiting 89. There is still much work to do to engage people most at risk that present higher levels of socioeconomic deprivation, more frequently current smokers, as they may be less willing to engage with healthcare interventions and constitute a particular challenge to secure an informed choice 89.
Response to recruitment to screening programmes in trial settings ranged from 1% to 5% of people approached with invitations and letters, and the adherence to screening decreased over time 9. In the UKLS trial, which used a true population approach, 31% of eligible people responded to an initial questionnaire but only 11.5% of participants were at high enough risk for trial entry and 47% of these gave their consent 89. In this trial, an analysis showed that female sex, older age, and more deprived socioeconomic background were independent determinants of reduced participation 89. In the NELSON trial, 32% of those eligible responded to a questionnaire on general health, lifestyle, and smoking history (which did not mention the trial), 19% of the respondents met the eligibility criteria and received an invitation to participation in the trial, an information leaflet, and an informed consent form combined with a short questionnaire. Of these individuals, 51% gave informed consent and were recruited 89.
In the UK, there has been a recent focus on pilot programmes to enhance informed participation in lung cancer screening within socioeconomically deprived areas. All of these pilots identified ever smokers as potentially at risk of lung cancer and the first contact was with the primary care doctor 89. One of these, the London-based Lung Screen Uptake Trial (LSUT) showed a participation rate of 53%, of which 91% of those eligible chose to be screened 89. A reminder letter providing a second prescheduled appointment increased the uptake of lung cancer screening among nonresponders by 24% 89.
The UK primary care system is proving to be an important tool in improving participation in screening pilots. The Liverpool Lung Health Check invited individuals with a documented history of smoking or diagnosis of COPD recorded in primary care records, an approach that led to 40% uptake in the first round of the pilot programme, which suggests that electronic primary care records can aid recruitment to screening and stresses the importance of accurate smoking data 97. In a recent review, interventions that were most effective in improving access to lung cancer screening targeted priority populations, raised community-level awareness, tailored materials for sociocultural acceptability, did not depend on prior patient engagement/registration with the health care system, proactively considered costs related to participation, and enhanced utilization through informed decision-making 98.
Final Remarks on LDCT Screening
In conclusion, there is strong evidence that LDCT lung cancer screening is effective in reducing lung cancer mortality burden. Considering smoking cessation remains the most important long-term intervention to decrease morbidity and mortality from lung cancer, this should be an integrating part of any lung cancer screening programme to be implemented.
Pulmonologists have a crucial role in identifying people eligible for lung cancer screening, involving family doctors, sharing the decision-making process and promoting tobacco cessation. Family doctors, although overloaded with administrative duties, will need to be persuaded to raise awareness of screening among high-risk patients and convey objective information about its risks and benefits. All physicians involved should work in close collaboration with psychologists and experts in tobacco-cessation interventions, as this will be critical to optimize the effectiveness of screening programs 30. The main eligibility criteria are summarized in Table 2.
Table 2 Summary of eligibility criteria for LDCT screening
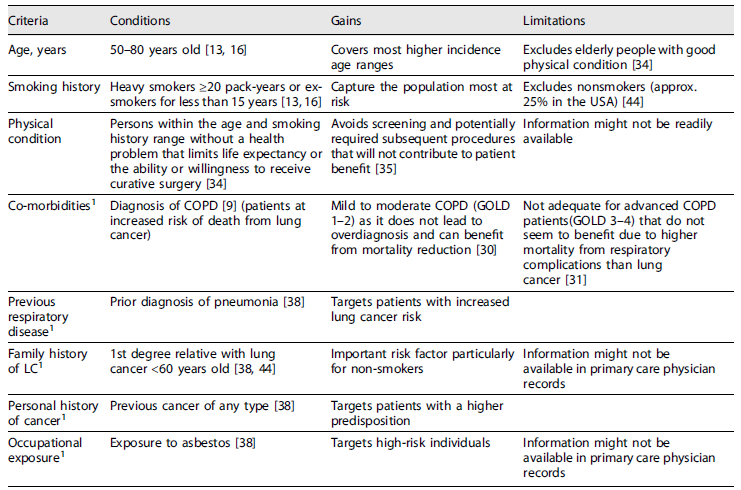
1Criteria included in multivariable risk calculation models that improve risk stratification and patient selection.
Recently, an Evidence Review Report produced and published by the Science Advice for Policy by European Academies (SAPEA) consortium for the European Commission to contribute to the improvement of cancer screening in the European Union, considered that there is scientific evidence for extending population-based screening programmes to additional types of cancers including lung cancer and considers that “high-quality CT screening can significantly reduce the burden of lung cancer in the EU, possibly to a similar extent to that achieved by current breast screening programmes” 99.
This report also indicates that “lung cancer screening should include high-risk current and ex-smokers of both sexes around ages 50-80, with eligibility based on a minimum number of pack-years smoked and/or a personalized risk score” and “Pilot projects and regular, timely monitoring and evaluation of quality indicators, process indicators and intermediate outcomes should be mandatory for all new lung cancer screening programmes” and should be accompanied by smoking cessation interventions to maximize the benefits for patients and increase cost-effectiveness 99. In the NLST, the NNS to prevent 1 lung cancer death (among those who completed at least 1 screening) was 320 and the NNS to prevent 1 death overall was 219 over 6.5 years. These benefits compare favorably with numbers needed to invite to screen to prevent 1 breast cancer death in mammography trials of 1,904 (95% CI: 929-6,378) for women aged 39-49 years, of 1,339 (95% CI: 322-7,455) for women aged 50-59 years, and of 377 (95% CI: 230-1,050) for women aged 60-69 years after 11-20 years of follow-up 13,100. They also compare with an NNS with a flexible sigmoidoscopy of 871 (95% CI: 567-1,874) to prevent 1 colorectal cancer death 13,101.
Nevertheless, there is a critical need for non-invasive and highly sensitive diagnostic methods to improve early diagnosis and outcomes 57. The specificity of a screening programme might be increased by the inclusion of nonimaging, noninvasive biomarkers including genetic tests and other laboratory predictors, to allow a better discrimination between benign versus malignant conditions 15,46. Body fluids, such as blood, are considered ideal samples for disease diagnosis 57. Breath tests for lung cancer screening through analysis of volatile organic compounds (VOCs) should also be considered a strong possibility for screening and are being tested in clinical trials 26,102.
New Screening Methods in Development
Novel screening technologies and biomarkers are rapidly growing and will likely gain wider acceptance once tested in well-designed clinical trials and can potentially be game-changers in lung cancer screening and diagnosis 61.
A biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of biological and pathogenic processes, or pharmacologic responses to therapeutic intervention” 103. Advances in molecular biology and bioinformatics have resulted in the identification of several potential biomarkers that could be relevant in the management of patients with lung cancer. Several different biomarkers have been evaluated in patients with lung cancer, including those that target early disease detection or detecting minimal residual disease, and those predicting both the rates of relapse and response to treatment 103. Biomarkers can be obtained from body fluids (including serum, plasma, urine, saliva, and sputum), epithelial cells of the respiratory tract, and exhaled breath 61,104.
If biomarkers were available to improve the sensitivity and specificity of lung cancer screening, they could inform strategies to reduce the risk of harm due to unnecessary downstream procedures 10. Additionally, available evidence shows that up to 30% of surgically treated stage I patients die from recurrence 105, therefore the evaluation of noninvasive or tissue-based biomarkers in patients after stage I tumor resection could also identify those at a high risk of recurrence, thus, leading to improved clinical management 103.
However, there are still many challenges that need to be overcome to enable exact diagnosis of lung cancer. High-sensitive and specific biomarkers are still pending discovery via genomic and proteomic profiling as well as the development of high-sensitive detection methods such as biosensors for lung cancer detection 106. Additionally, due to the low concentration levels of lung cancer biomarkers, the sensitivity, specificity, and stability of biosensors are critical in the early diagnosis of lung cancer 103,106.
A biosensor is commonly defined as a self-contained small analytical device that combines a biological recognition system and a physiochemical transducer for the detection of target molecules by converting the recognition signal into a detectable output signal 107. Generally, biosensors can be categorized as electrochemical, optical and mass-based, if signal transduction is respectively based on an electrochemical reaction at an electrode surface, biorecognition with an optical transducer, and through detection of changes in mass 107. The biomarkers of lung cancer are mainly divided into two categories: DNA/genetic-based biomarkers and protein-based biomarkers 104.
DNA/Genetic-Based Biomarkers
The development of lung cancer results from the interaction between genetic mutations and epigenetic alterations, but the exact mechanisms are not completely understood. Epigenetic changes are defined as heritable modifications in gene expression which do not alter the DNA coding sequence directly. Epigenetic changes observed in oncogenesis consist of aberrant DNA methylation patterns, histone modifications, and regulation by small noncoding RNAs (microRNAs or miRNAs) 108.
DNA methylation is an important epigenetic change that can affect gene expression without changing the DNA sequence, may serve as a biomarker for noninvasive diagnosis, and is useful in the early diagnosis of cancer 109. However, detecting aberrant methylation of a single-gene biomarker is not enough to diagnose cancer 109,110. MiRNAs are endogenous single-stranded non-coding small RNAs with a length of ∼22 nucleotides that have important roles in gene silencing 103,107.
The action of these small molecules is to block or degrade mRNA molecules and suppress their expression stopping protein production 107. An increasing number of miRNAs have been reported to be deregulated in the early stage of cancers, sometimes before any clinical symptoms and imaging evidence 103. Some experiments assessing different microRNAs showed 92-100% specificity and 75-85% sensitivity in the distinction between lung cancer and normal cases 107,111. The presence of microRNA molecules in body fluids indicates their potential as biomarkers for cancer diagnosis 107.
Protein-Based Biomarkers
Proteomics may be defined as the large-scale characterization of proteins expressed by the genome 112 involving the separation, identification, and quantification of proteins 103. Proteomics is expected to complement gene analyses for evaluating disease development, prognosis, and response to treatment because proteome analysis can provide the link between gene sequence and cellular physiology 113. A variety of serologic markers have been proposed for lung cancer diagnosis and prognosis, including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA 19-9), cancer antigen 12-5 (CA125), neuron-specific enolase (NSE), and cytokeratin 19 fragment 21-1 (CYFRA21-1) 114. However, most of these markers only showed better sensitivity in advanced stages of lung cancer, are primarily used for monitoring disease progression and tend to show suboptimal diagnostic values for lung cancer with sensitivity values usually below 50% 114,115. As such, they are not adequate for early diagnosis of lung cancer 116-118. Nevertheless, the combination of multiple markers instead of a single one can significantly improve both the sensitivity and the specificity 119.
Proteomics has been widespread in the fields of life sciences and medicine as an important part of post-genomics era research; however, it also has some difficulties compared to genomics 113. Among these are the diversity of proteins, proteome dynamics lower stability and the fact that determination of proteins is affected by time and space 113.
Liquid Biopsy for Cancer Screening
Considering the fast development in this field all the different liquid biopsy methods must be standardized and validated to support harmonization of protocols and quality assurance 99. The newly founded European Liquid Biopsy Society (ELBS) is working to bring the process of liquid biopsy into clinical practice through efforts to join together people and institutions from all over Europe and beyond united by the common interest to translate the promising clinical research results in effective benefit for cancer patients 120. Another key player is the International Liquid Biopsy Standardization Alliance (ILSA) which “comprises organizations that recognize the importance of working toward the global use of liquid biopsy and common reference standards in oncology and seek to promote their use in the broader medical community” and are aligned in a common vision of liquid biopsy’s use “to support clinical decision making and regulatory considerations, which will ultimately reduce the need for unnecessary and invasive solid tumor biopsies” 121.
Recent publications and reviews highlight that the detection of circulating tumor cells (CTCs), circulating miRNAs, exosomes, circulating free DNA, or proteins in plasma, may be useful for uncovering early stage lung cancer 103. These circulating biomarkers could add value in the diagnosis of lung cancer when one or several nodules of uncertain malignancy are observed on a chest CT scan or might help in the prediction of lung cancer onset in higher risk populations, such as heavy smokers, patients with COPD and patients over 55 years of age 103.
Circulating Tumor Cells
Circulating tumor cells (CTCs) refer to the cancer cells that have escaped from the primary tumor and disseminated into the bloodstream or lymphatic system. They can spread to other organs and give rise to metastatic tumors and also cause tumor recurrence. Detection of CTCs can assist with the discrimination between benign and malignant pulmonary nodules 122.
A study conducted in one center in France on patients with COPD showed that CTCs may be detected in the absence of a cancer nodule detectable by a CT scan and be a hallmark of a developing invasive cancer 123. COPD is an independent risk factor for lung cancer. Migration of CTCs into the bloodstream is an early event that occurs during carcinogenesis. CTCs were detected in 3% of COPD patients (5 out of 168 patients), but the CT scan performed at the same time as the blood filtration failed to show lung nodules. The annual surveillance of CTC-positive patients by CT-scan screening detected lung nodules 1-4 years after CTC detection, leading to prompt surgical resection and histopathological diagnosis of early stage lung cancer. No CTCs were detected in controlled smoking and nonsmoking healthy individuals 123.
Based on these preliminary results, a consortium of 21 university centers in France implemented the AIR Project, which was a prospective, multicentre, double-blinded, cohort study that aimed to include at least 600 patients. The primary objective was to determine the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of CTCs for the early detection of lung cancer in a cohort of asymptomatic participants at high risk for LC, which include, smokers and ex-smokers (≥30 pack-years, who quitted ≤15 years), aged ≥55 years, with COPD. The study participants would undergo yearly screening rounds for 3 years plus a 1-year follow-up. Each round included LDCT plus peripheral blood sampling for CTC detection (ClinicalTrials.gov, NCT02500693) 124. The estimated study completion date was December 2019 but no results were formally posted. However, a 2020 publication from Marquette et al. 125 concluded that CTC detection using the applied technique (ISET, isolation by size of epithelial tumor cell technique) is not sufficiently reliable to be recommended for use in lung cancer screening. Although the study results were disappointing with the method used for CTC detection, they do not imply that the results would have been the same if another method had been used 125.
The detection of CTCs cannot replace CT scan or biopsy for the diagnosis of patients with suspicious malignant lung lesions. However, the detection of CTCs has the potential to provide more information about pulmonary nodules and can be of value in helping clinicians to decide on the appropriate treatment for pulmonary nodules 122.
Plasma miRNA
Detection of circulating miRNAs such as serum miRNAs can help diagnose various malignancies, and can also be a potential diagnostic marker for early NSCLC patients 126. They have several advantages over some other markers of cancer detection namely, high stability in cell-free fluids even in non-ideal handling conditions, can be measured repeatedly over time in a noninvasive manner, can be used to predict cancer in high-risk populations years in advance and cost of analysis is relatively low 115.
A retrospective analysis of a miRNA signature in plasma from patients enrolled in the randomized Multicenter Italian Lung Detection (MILD) trial revealed a significant diagnostic performance for early detection of lung cancer 127,128. For their analysis, investigators developed a specific miRNA signature classifier (MSC) algorithm grouping patients into low, intermediate, and high risk of cancer-based on a predefined cutoff ratio value of 24 miRNAs identified from a previous training set. Each group was then examined for lung cancer occurrence, death and tumor stage 129. Comparative diagnostic performance was similar for MSC and LDCT. Combinations of both MSC and LDCT resulted in a five-fold reduction of LDCT false-positive rate to 3.7%, and therefore MSC could complement LCDT screening 127,128. Additionally, plasma miRNAs differentiated malignant from benign nodules potentially providing a noninvasive diagnosis of lung cancer among individuals with solitary pulmonary nodes and could be further evaluated in clinical trials 128,130. Circulating miRNAs in serum include two forms: serum miRNAs and serum exosomal miRNAs 126.
Exosomes are 30-100 nm extracellular vesicles consisting of nucleotides and proteins, secreted by specific cell types and are found in various body fluids 131,132. Exosomes can mediate cell-to-cell communication in both physiologic and pathologic processes 133. Due to the protection of the lipid bilayer, exosomal miRNAs have higher stability compared with serum miRNAs 126. Serum exosomal miRNA might be preferable biomarkers for patients with NSCLC at early stages, and a combination of serum miRNAs with serum exosomal miRNAs can contribute to further improvement of early diagnosis for NSCLC 126. It is of major importance to conduct a rigorous independent validation of the identified promising miRNA algorithms or their combination with other biomarkers in prospective screening cohorts 115.
Circulating-Free DNA
A strong link has been shown between some genomic alterations detected from circulating-free DNA (cfDNA) and the presence of early stage lung carcinoma 103. Tumor cells release DNA into the serum or plasma via multiple mechanisms, allowing detection of cancer-associated genetic alterations including point mutations, copy number variations, chromosomal rearrangements, and epigenetic aberrations 134. Detecting genetic and epigenetic biomarkers in cfDNA has emerged as a promising noninvasive approach for the diagnosis, prognosis, and treatment of cancer. Epigenetic alterations, especially for aberrant DNA methylation processes, contribute to tumor initiation and progression 134. It has been reported that about 25% of patients with stage I lung cancer will have recurrent disease due to occult metastasis 135. These biomarkers may also have the potential value to classify early stage lung cancers into subtypes with low-risk and high-risk recurrence and enable the appropriate treatment, such as the post-surgery adjuvant therapy that should mandatorily be given to patients with a higher risk of metastasis 134.
Some results from clinical trials that validate the screening potential of circulating ctDNA tests are available and others will be in the near future. Below, a few of them will be briefly highlighted.
CancerSEEK is a multi-analyte blood test that can detect eight common cancer types through assessment of the levels of circulating proteins and mutations in cell-free DNA. The test can identify the presence of relatively early cancers but also to localize the organ of origin of these cancers. CancerSEEK was applied to 1,005 patients with non-metastatic, clinically detected cancers of the ovary, liver, stomach, pancreas, esophagus, colorectal, lung, or breast. The median sensitivity of CancerSEEK was 73% for the most common stage evaluated (stage II), similar (78%) for stage III cancers, and lower (43%) for stage I cancers. The specificity was greater than 99%. In addition, CancerSEEK localized the cancer to a small number of anatomic sites in a median of 83% of the patients 136.
Detecting cancers Earlier Through Elective mutation-based blood Collection and Testing (DETECT-A) was a prospective, interventional study that included 10,006 women not previously known to have cancer. The study evaluated the feasibility and safety of multi-cancer blood testing (an early version of the CancerSEEK test) coupled with PET-CT imaging to detect cancer. Positive blood tests were independently confirmed by a diagnostic PET-CT, which also localized the cancer. Of a total of 96 cancers detected, 26 cancers were detected by blood testing alone. 1.0% of participants underwent PET-CT imaging based on false-positive blood tests, and 0.22% underwent a futile invasive diagnostic procedure. These data demonstrate that multi-cancer blood testing combined with PET-CT can be safely incorporated into routine clinical care, in some cases leading to surgery with the intent to cure 99,137. One of the key safety features of DETECT-A was the diagnostic PET-CT for confirmation of blood testing and localization of suspected cancers 137.
In 2021, NHS England launched the NHS-Galleri trial of a new blood test (called Galleri™) that can detect more than 50 types of asymptomatic cancers. This randomized control trial aims to see if using the Galleri test alongside existing cancer screening can help find cancer early. The test works by finding chemical changes in fragments of cfDNA that leak from tumors into the bloodstream. The trial recruited around 140,000 volunteers aged 50-77 and the initial results are expected by 2023. If successful the plan is to roll out the test to an additional 1 million people in 2024 and 2025 138.
The Screening for High Frequency Malignant Disease (SHIELD) study is a prospective, observational, multisite basket design trial without randomization. The primary objective of the study was to evaluate the sensitivity and specificity of a blood-based Guardant LUNAR-2 test to detect high-frequency cancer in screen-relevant populations. LUNAR-2 is a multimodal ctDNA blood test. The SHIELD study will recruit patients in separate cancer-risk cohorts with specified pathways for cancer screening and the first cohort to be recruited is for lung cancer screening. In this cohort, individuals at high risk of lung cancer will undergo standard-of-care screening with LDCT as per USPSTF guidelines and an investigational blood draw will be taken from all subjects enrolled that consent to do so. The study started in January 2022 and the estimated primary completion date in 2024 139.
Another study aims to evaluate the ability of cell-free circulating tumor DNA (ctDNA) using Guardant Health’s LUNAR technology to detect early stage lung cancer as compared to healthy volunteers and those patients at high risk for lung cancer but without a lung cancer diagnosis. The primary outcome measures are the sensitivity and specificity of the assay as well as its prospective negative predictive value. The study which is currently in the recruitment phase, started in December 2017 and the estimated completion date is December 2023 140.
In the next few years, the evidence that will be available from the multiplicity of ongoing studies with genetic and protein markers in body fluids is likely to enable optimization of lung cancer screening programs and expand its application. These new screening approaches have the potential to be valid diagnostic tools for early lung cancer detection provided an adequate standardization of the methodologies is secured to allow the analysis of large amounts of data.
Breath Analysis for Lung Cancer Screening
Human exhaled breath contains several hundreds of VOCs that can be seen as a fingerprint that could be used to differentiate between healthy individuals and cancer patients 141. Lung cancer causes oxidative stress and induces oxidase enzymes that produce higher concentrations of specific VOCs in exhaled breath 142. In particular, the presence of alkanes and benzene derivatives in the exhaled gases present typical features for a diagnosis of lung cancer 60,143. The potential use of VOCs in early diagnosis of cancer is interesting due to its low level of invasiveness, low cost, and relative ease of implementation on a large scale 141,144,145. However, a challenge of breath analysis lies in the very low concentration (from nanomolar to picomolar) of VOCs that require pre-concentration steps to ensure proper analysis 141.
The approaches used to analyze the exhaled breath have demonstrated high sensitivity and specificity for lung cancer diagnosis, and include detection of breath print by electronic nose or integrated analysis of a wide range of VOCs detected by gas chromatography/mass spectrometry 145. Diagnostics using an electronic nose is simple, sufficiently accurate, inexpensive, noninvasive, allows online diagnosis and can differentiate heterogeneous disorders. The electronic nose is an instrument made up of chemical sensors combined with a pattern recognition system. The measurement is based on electrical resistance, ion-gas or colorimetric sensor response 145. However, a significant challenge to exhaled breath analysis by electronic nose is the heterogeneity of patients 145.
Currently, the Lung Cancer Indicator Detection (LuCID) study investigates the diagnostic accuracy of Field Asymmetric Ion Mobility Spectrometry (FAIMS) for diagnosis of lung cancer by analysis of exhaled VOCs. LuCID study aims to validate the use of a breath analysis in a population of patients clinically suspected of having lung cancer who consent to participate. LuCID is an international, multi-center case-control study. The procedure is noninvasive and will require the patient to breathe normally into a facemask to collect 2.5 L of breath amounting to approximately 10 min of breathing. The resulting samples will be analyzed for VOCs by Gas Chromatography coupled to Mass Spectrometry and Gas Chromatography coupled to Field Asymmetrical Ion Mobility Spectrometry. The resulting VOC profiles will be used to generate a diagnostic algorithm to differentiate between patients with and without lung cancer. This will form the foundation for a subsequent study in a population at risk for the development of lung cancer. If sufficiently accurate for early-stage disease, analysis of breath VOCs could help implement large-scale screening for lung cancer. The estimated study completion date was March 2022 but so far no results have been published 102. A summary of current and future screening methods is included in Table 3.
Table 3 Summary of lung cancer screening methods
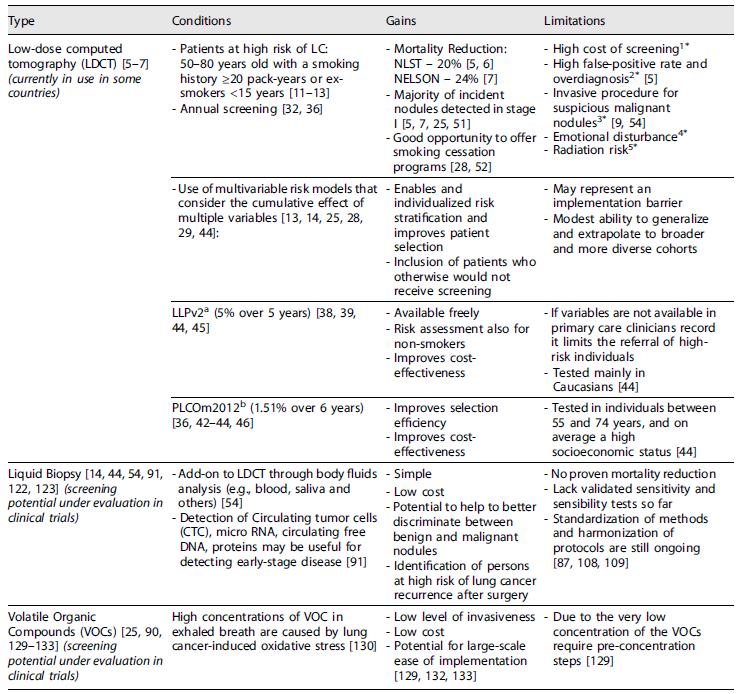
*Current mitigation measures for the above limitations.1There is available evidence supporting LDCT screening is cost-effective 29,70,71,73-76 and concomitant smoking cessation programs, incorporating coronary artery calcification score and emphysema assessment can improve cost-effectiveness 69,72,73,78.2Nodule management procedures using nodule volume and volume doubling time instead of only nodule diameter reduced false-positives 14,25,32,64.3Circulating biomarkers can add value through discrimination between potentially benign and malignant nodules 91.4Psychological support and proactive communication about atypical findings and probability of cancer 25.5Radiation risk is likely overestimated and will decrease using ultra-LDCT 25.aModified LLP model.bProstate, lung, colorectal, and ovarian cancer screening trial-modified model.
Conclusions
There is strong evidence that LDCT lung cancer screening is effective in reducing lung cancer mortality burden. Additionally, the current knowledge of the patients’ eligibility criteria and mechanisms to mitigate the potential risks support its prompt implementation. Nevertheless, there is a critical need for noninvasive and highly sensitive diagnostic methods to improve early diagnosis, and outcomes that can be used as a complement to LDCT, to allow better discrimination between benign versus malignant conditions, or even replace LDCT in the future.
Statement of Ethics
In conducting this literature review, we adhered to the highest ethical standards to ensure the integrity and reliability of our findings. All information obtained from literature sources was subject to proper citation and acknowledgment. We strived to conduct our review with impartiality and objectivity, ensuring a balanced representation of diverse viewpoints and perspectives. Authors were credited for their contributions, and any potential conflicts of interest were disclosed.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Teresa Guerreiro was responsible for the concept, literature review, and writing of the manuscript, and Pedro Aguiar and António Araújo for the critical revision of the manuscript.
Data Availability Statement
The manuscript is a review article that is part of Teresa Guerreiro’s PhD thesis. The content of the manuscript will be publicly available after the thesis submission and public discussion. This study adheres to the principles of data transparency and accessibility, in alignment with the journal’s data sharing policies.














